Abstract
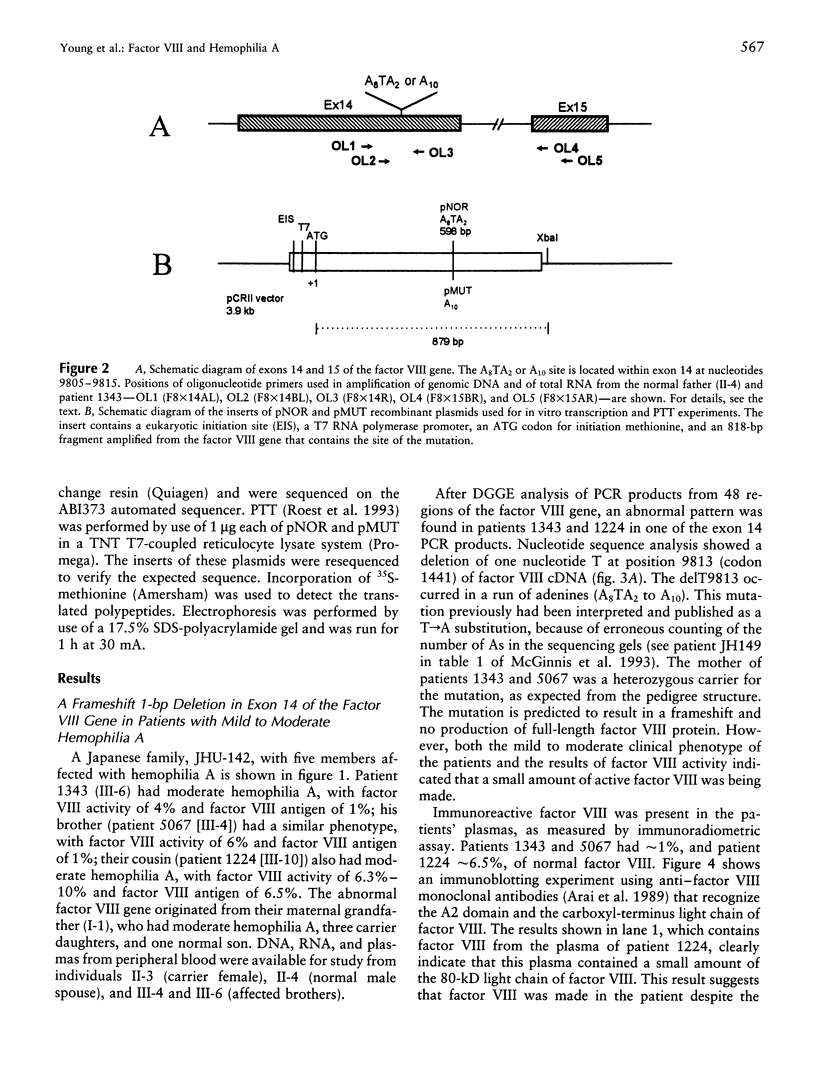
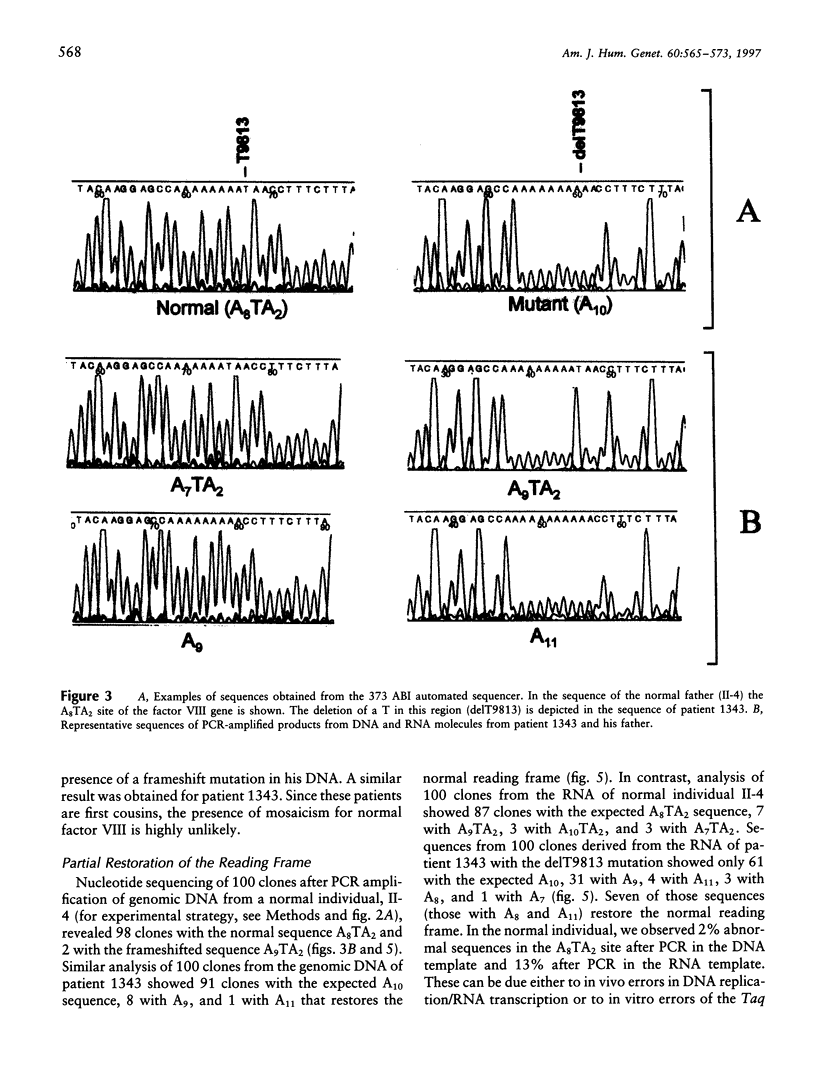
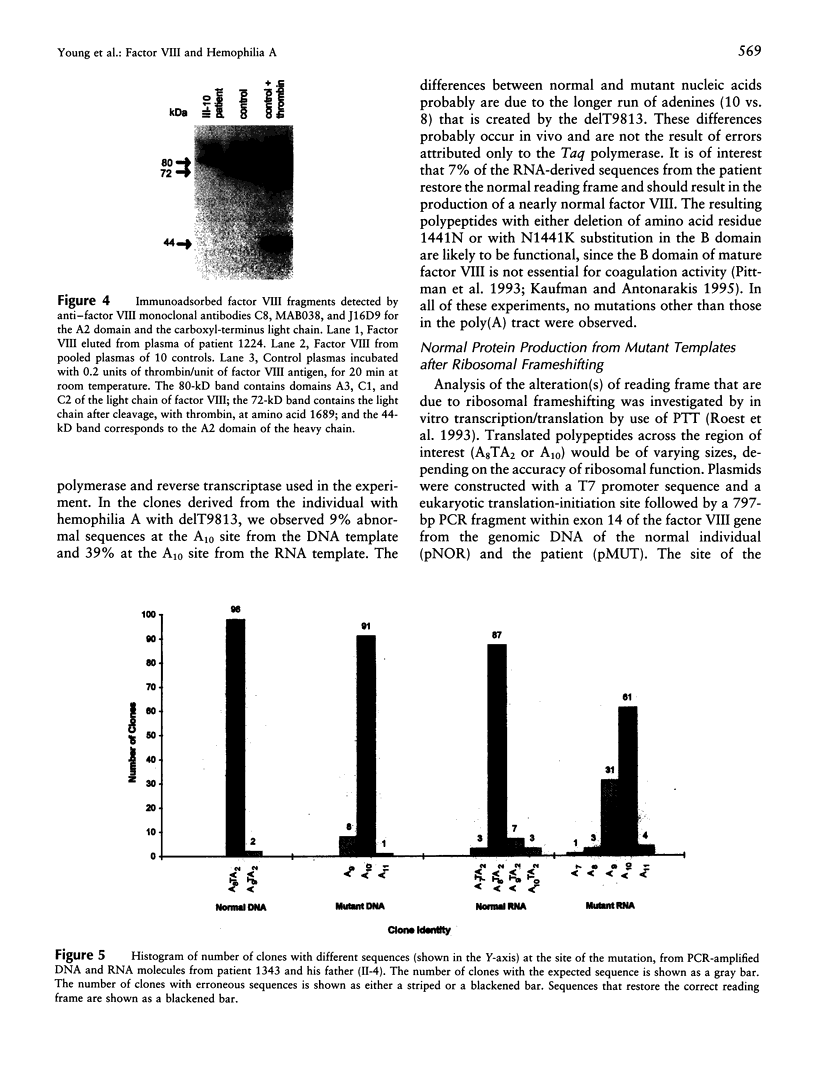
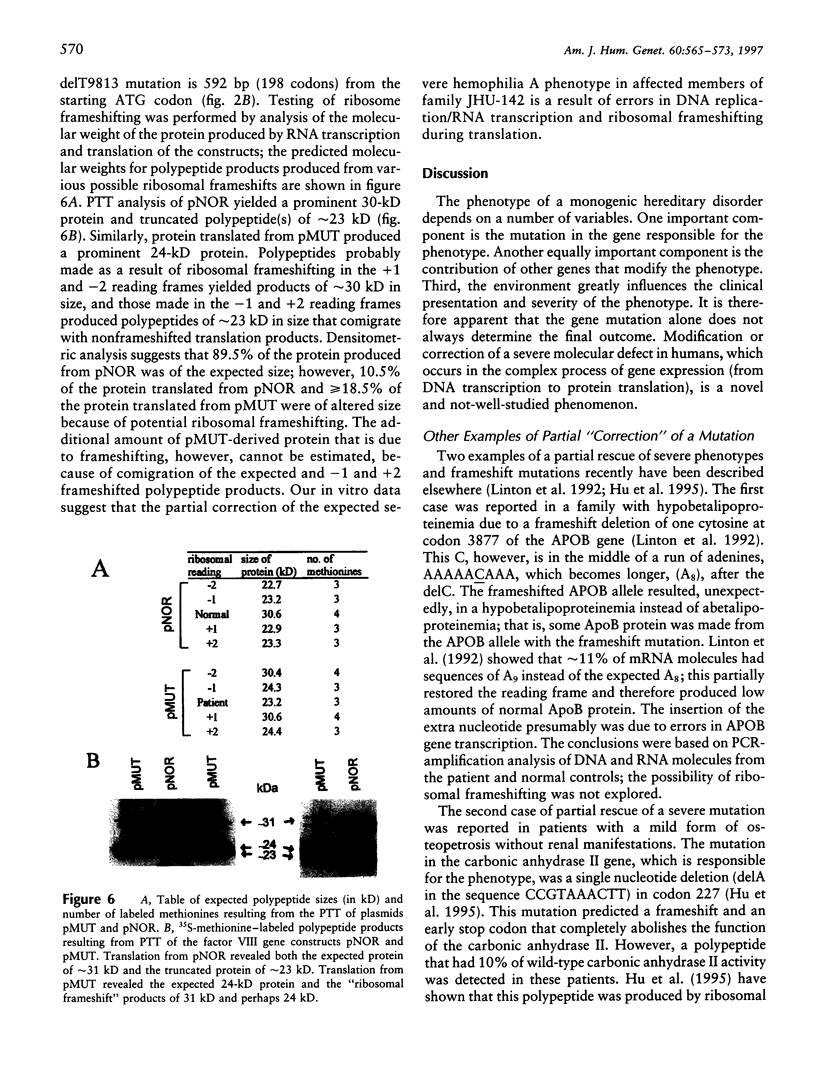
Although the molecular defect in patients in a Japanese family with mild to moderately severe hemophilia A was a deletion of a single nucleotide T within an A8TA2 sequence of exon 14 of the factor VIII gene, the severity of the clinical phenotype did not correspond to that expected of a frameshift mutation. A small amount of functional factor VIII protein was detected in the patient's plasma. Analysis of DNA and RNA molecules from normal and affected individuals and in vitro transcription/translation suggested a partial correction of the molecular defect, because of the following: (i) DNA replication/RNA transcription errors resulting in restoration of the reading frame and/or (ii) "ribosomal frameshifting" resulting in the production of normal factor VIII polypeptide and, thus, in a milder than expected hemophilia A. All of these mechanisms probably were promoted by the longer run of adenines, A10 instead of A8TA2, after the delT. Errors in the complex steps of gene expression therefore may partially correct a severe frameshift defect and ameliorate an expected severe phenotype.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Kazazian H. H., Tuddenham E. G. Molecular etiology of factor VIII deficiency in hemophilia A. Hum Mutat. 1995;5(1):1–22. doi: 10.1002/humu.1380050102. [DOI] [PubMed] [Google Scholar]
- Antonarakis S. E., Rossiter J. P., Young M., Horst J., de Moerloose P., Sommer S. S., Ketterling R. P., Kazazian H. H., Jr, Négrier C., Vinciguerra C. Factor VIII gene inversions in severe hemophilia A: results of an international consortium study. Blood. 1995 Sep 15;86(6):2206–2212. [PubMed] [Google Scholar]
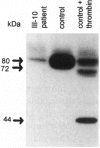
- Arai M., Inaba H., Higuchi M., Antonarakis S. E., Kazazian H. H., Jr, Fujimaki M., Hoyer L. W. Direct characterization of factor VIII in plasma: detection of a mutation altering a thrombin cleavage site (arginine-372----histidine). Proc Natl Acad Sci U S A. 1989 Jun;86(11):4277–4281. doi: 10.1073/pnas.86.11.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J. F., Elseviers D., Gorini L. Low activity of -galactosidase in frameshift mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1192–1195. doi: 10.1073/pnas.69.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank A., Gallant J. A., Burgess R. R., Loeb L. A. An RNA polymerase mutant with reduced accuracy of chain elongation. Biochemistry. 1986 Oct 7;25(20):5920–5928. doi: 10.1021/bi00368a013. [DOI] [PubMed] [Google Scholar]
- Brierley I. Ribosomal frameshifting viral RNAs. J Gen Virol. 1995 Aug;76(Pt 8):1885–1892. doi: 10.1099/0022-1317-76-8-1885. [DOI] [PubMed] [Google Scholar]
- Chamorro M., Parkin N., Varmus H. E. An RNA pseudoknot and an optimal heptameric shift site are required for highly efficient ribosomal frameshifting on a retroviral messenger RNA. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):713–717. doi: 10.1073/pnas.89.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M., Fayet O. Translational frameshifting in the control of transposition in bacteria. Mol Microbiol. 1993 Feb;7(4):497–503. doi: 10.1111/j.1365-2958.1993.tb01140.x. [DOI] [PubMed] [Google Scholar]
- Clare J. J., Belcourt M., Farabaugh P. J. Efficient translational frameshifting occurs within a conserved sequence of the overlap between the two genes of a yeast Ty1 transposon. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6816–6820. doi: 10.1073/pnas.85.18.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam E., Pleij K., Draper D. Structural and functional aspects of RNA pseudoknots. Biochemistry. 1992 Dec 1;31(47):11665–11676. doi: 10.1021/bi00162a001. [DOI] [PubMed] [Google Scholar]
- Economou E. P., Bergen A. W., Warren A. C., Antonarakis S. E. The polydeoxyadenylate tract of Alu repetitive elements is polymorphic in the human genome. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2951–2954. doi: 10.1073/pnas.87.8.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower A. M., McHenry C. S. The gamma subunit of DNA polymerase III holoenzyme of Escherichia coli is produced by ribosomal frameshifting. Proc Natl Acad Sci U S A. 1990 May;87(10):3713–3717. doi: 10.1073/pnas.87.10.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitschier J., Wood W. I., Goralka T. M., Wion K. L., Chen E. Y., Eaton D. H., Vehar G. A., Capon D. J., Lawn R. M. Characterization of the human factor VIII gene. Nature. 1984 Nov 22;312(5992):326–330. doi: 10.1038/312326a0. [DOI] [PubMed] [Google Scholar]
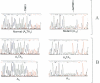
- Higuchi M., Antonarakis S. E., Kasch L., Oldenburg J., Economou-Petersen E., Olek K., Arai M., Inaba H., Kazazian H. H., Jr Molecular characterization of mild-to-moderate hemophilia A: detection of the mutation in 25 of 29 patients by denaturing gradient gel electrophoresis. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8307–8311. doi: 10.1073/pnas.88.19.8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Kazazian H. H., Jr, Kasch L., Warren T. C., McGinniss M. J., Phillips J. A., 3rd, Kasper C., Janco R., Antonarakis S. E. Molecular characterization of severe hemophilia A suggests that about half the mutations are not within the coding regions and splice junctions of the factor VIII gene. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7405–7409. doi: 10.1073/pnas.88.16.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P. Y., Waheed A., Sly W. S. Partial rescue of human carbonic anhydrase II frameshift mutation by ribosomal frameshift. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2136–2140. doi: 10.1073/pnas.92.6.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Varmus H. E. Expression of the Rous sarcoma virus pol gene by ribosomal frameshifting. Science. 1985 Dec 13;230(4731):1237–1242. doi: 10.1126/science.2416054. [DOI] [PubMed] [Google Scholar]
- Jacques J. P., Kolakofsky D. Pseudo-templated transcription in prokaryotic and eukaryotic organisms. Genes Dev. 1991 May;5(5):707–713. doi: 10.1101/gad.5.5.707. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Alexander P. S. The base substitution fidelity of eucaryotic DNA polymerases. Mispairing frequencies, site preferences, insertion preferences, and base substitution by dislocation. J Biol Chem. 1986 Jan 5;261(1):160–166. [PubMed] [Google Scholar]
- Kunkel T. A. The mutational specificity of DNA polymerases-alpha and -gamma during in vitro DNA synthesis. J Biol Chem. 1985 Oct 15;260(23):12866–12874. [PubMed] [Google Scholar]
- Lakich D., Kazazian H. H., Jr, Antonarakis S. E., Gitschier J. Inversions disrupting the factor VIII gene are a common cause of severe haemophilia A. Nat Genet. 1993 Nov;5(3):236–241. doi: 10.1038/ng1193-236. [DOI] [PubMed] [Google Scholar]
- Lazarchick J., Hoyer L. W. Immunoradiometric measurement of the factor VIII procoagulant antigen. J Clin Invest. 1978 Nov;62(5):1048–1052. doi: 10.1172/JCI109209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton M. F., Pierotti V., Young S. G. Reading-frame restoration with an apolipoprotein B gene frameshift mutation. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11431–11435. doi: 10.1073/pnas.89.23.11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S., Wang J., Myeroff L., Parsons R., Sun L., Lutterbaugh J., Fan R. S., Zborowska E., Kinzler K. W., Vogelstein B. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995 Jun 2;268(5215):1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- McGinniss M. J., Kazazian H. H., Jr, Hoyer L. W., Bi L., Inaba H., Antonarakis S. E. Spectrum of mutations in CRM-positive and CRM-reduced hemophilia A. Genomics. 1993 Feb;15(2):392–398. doi: 10.1006/geno.1993.1073. [DOI] [PubMed] [Google Scholar]
- Pittman D. D., Alderman E. M., Tomkinson K. N., Wang J. H., Giles A. R., Kaufman R. J. Biochemical, immunological, and in vivo functional characterization of B-domain-deleted factor VIII. Blood. 1993 Jun 1;81(11):2925–2935. [PubMed] [Google Scholar]
- Roest P. A., Roberts R. G., Sugino S., van Ommen G. J., den Dunnen J. T. Protein truncation test (PTT) for rapid detection of translation-terminating mutations. Hum Mol Genet. 1993 Oct;2(10):1719–1721. doi: 10.1093/hmg/2.10.1719. [DOI] [PubMed] [Google Scholar]
- Rossiter J. P., Young M., Kimberland M. L., Hutter P., Ketterling R. P., Gitschier J., Horst J., Morris M. A., Schaid D. J., de Moerloose P. Factor VIII gene inversions causing severe hemophilia A originate almost exclusively in male germ cells. Hum Mol Genet. 1994 Jul;3(7):1035–1039. doi: 10.1093/hmg/3.7.1035. [DOI] [PubMed] [Google Scholar]
- Sekine Y., Ohtsubo E. Frameshifting is required for production of the transposase encoded by insertion sequence 1. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4609–4613. doi: 10.1073/pnas.86.12.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchihashi Z., Kornberg A. Translational frameshifting generates the gamma subunit of DNA polymerase III holoenzyme. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2516–2520. doi: 10.1073/pnas.87.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuddenham E. G., Schwaab R., Seehafer J., Millar D. S., Gitschier J., Higuchi M., Bidichandani S., Connor J. M., Hoyer L. W., Yoshioka A. Haemophilia A: database of nucleotide substitutions, deletions, insertions and rearrangements of the factor VIII gene, second edition. Nucleic Acids Res. 1994 Sep;22(17):3511–3533. doi: 10.1093/nar/22.17.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner L. A., Weiss R. B., Driscoll R., Dunn D. S., Gesteland R. F. Transcriptional slippage occurs during elongation at runs of adenine or thymine in Escherichia coli. Nucleic Acids Res. 1990 Jun 25;18(12):3529–3535. doi: 10.1093/nar/18.12.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]