Abstract
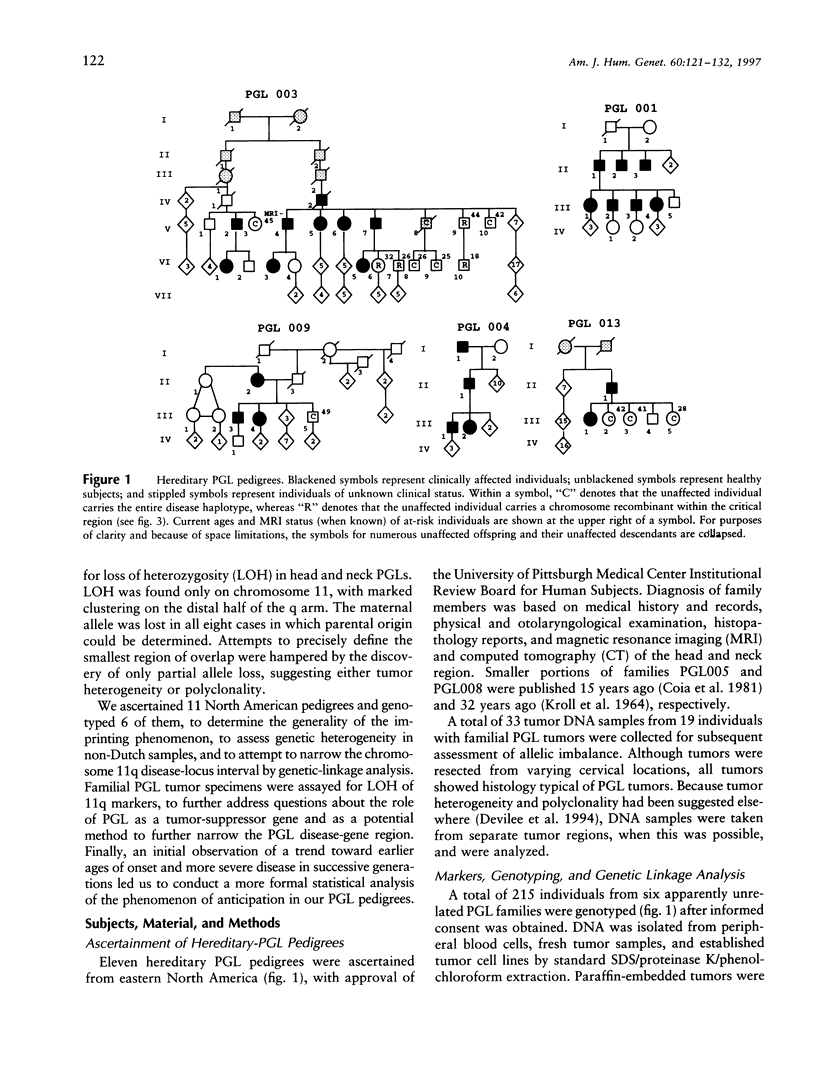
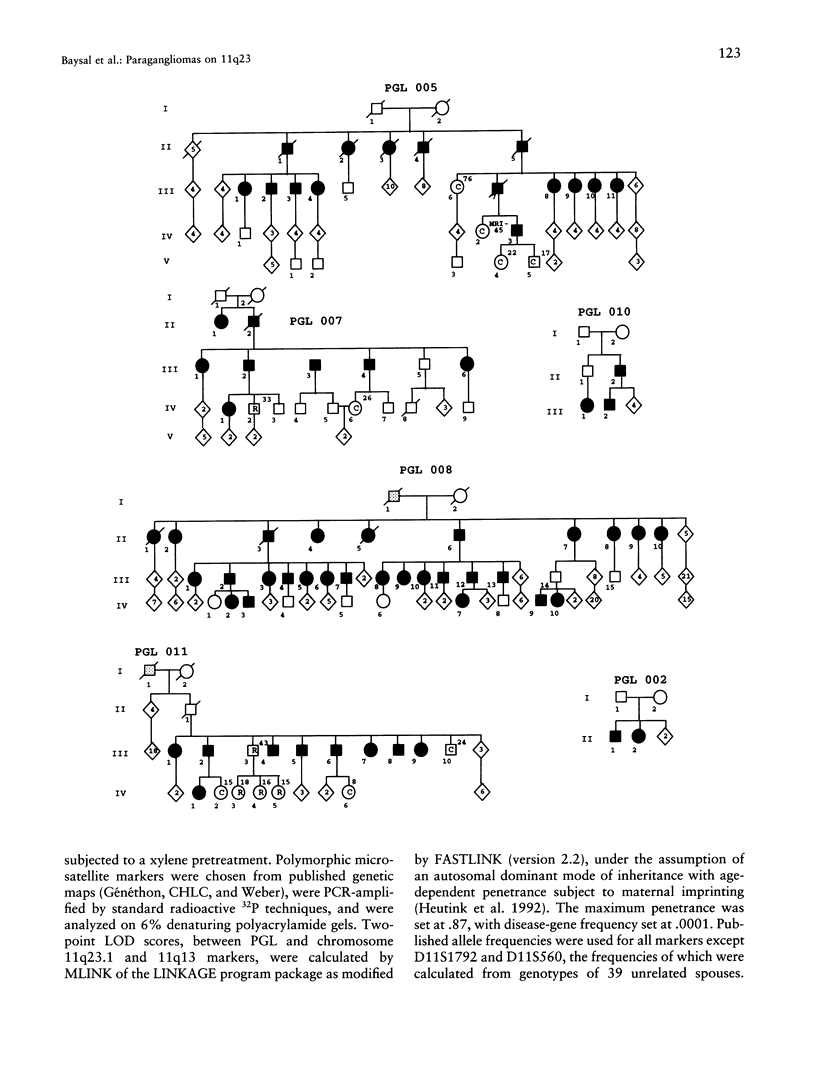
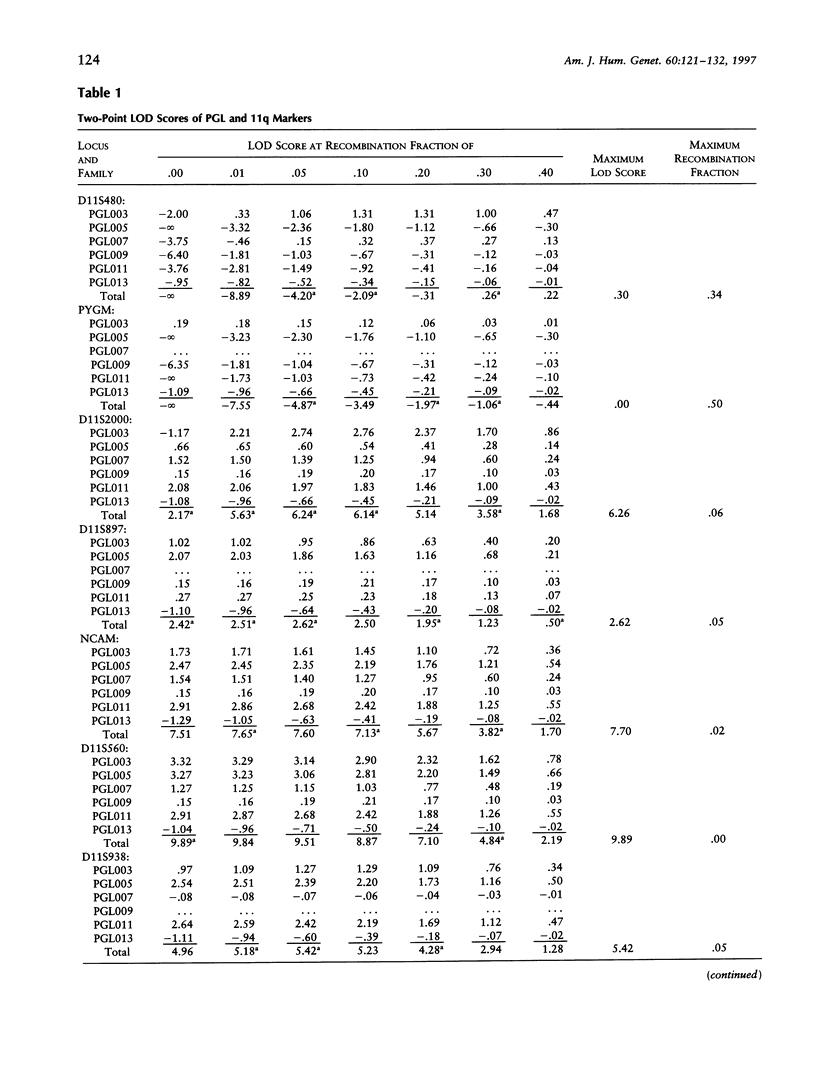
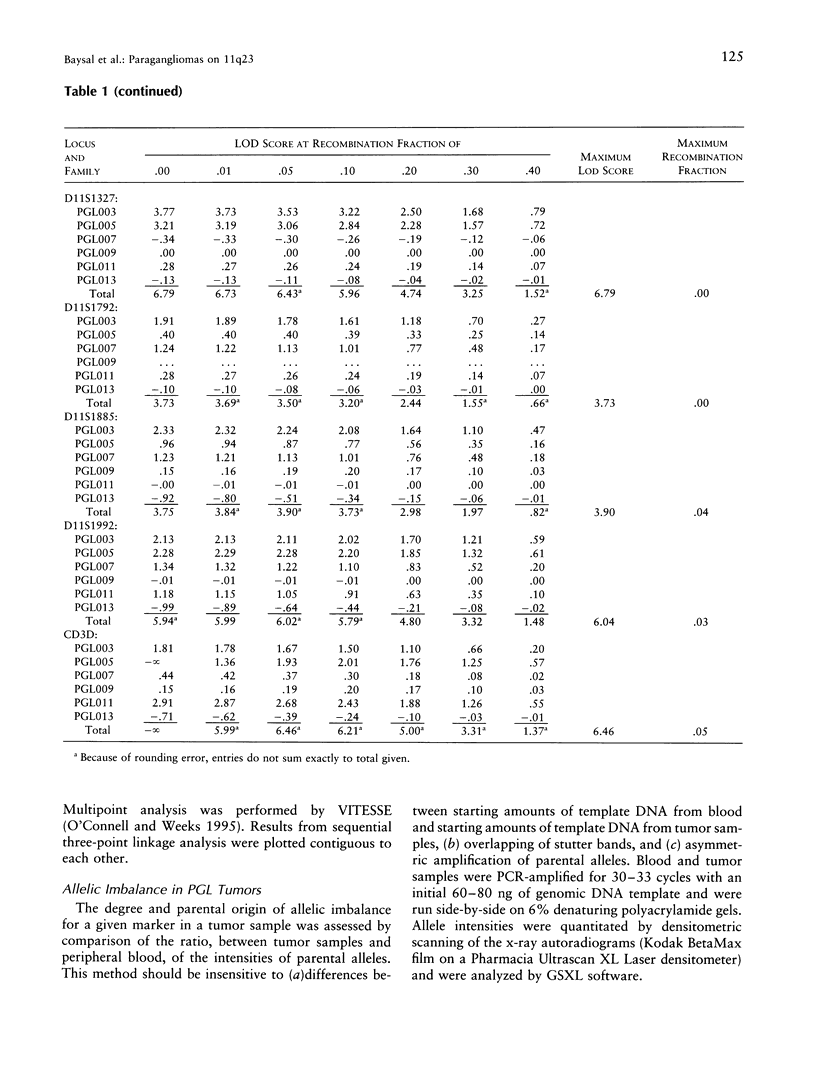
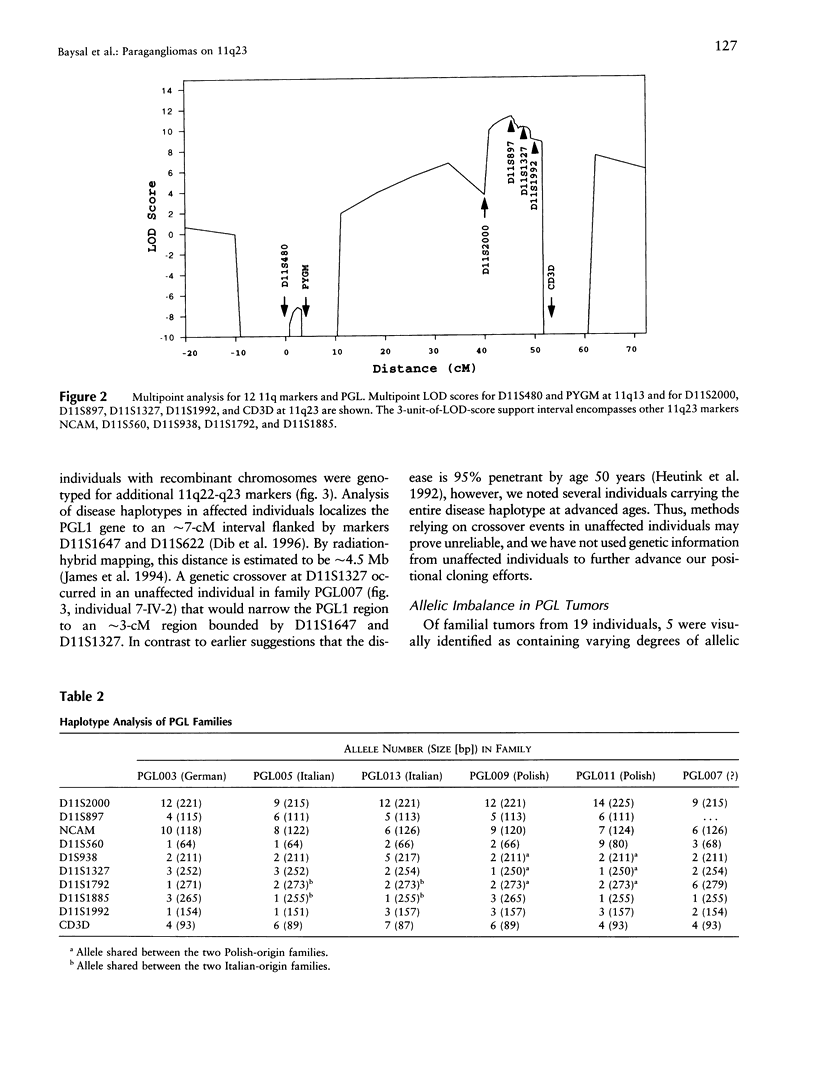
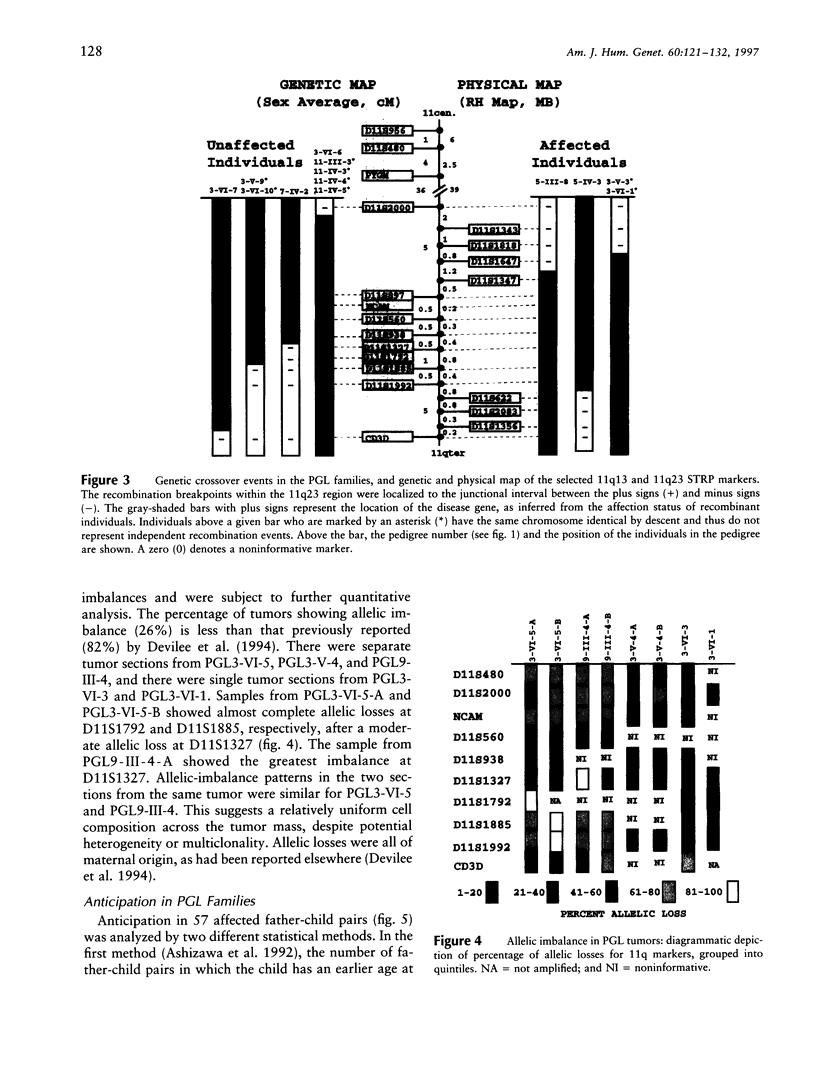
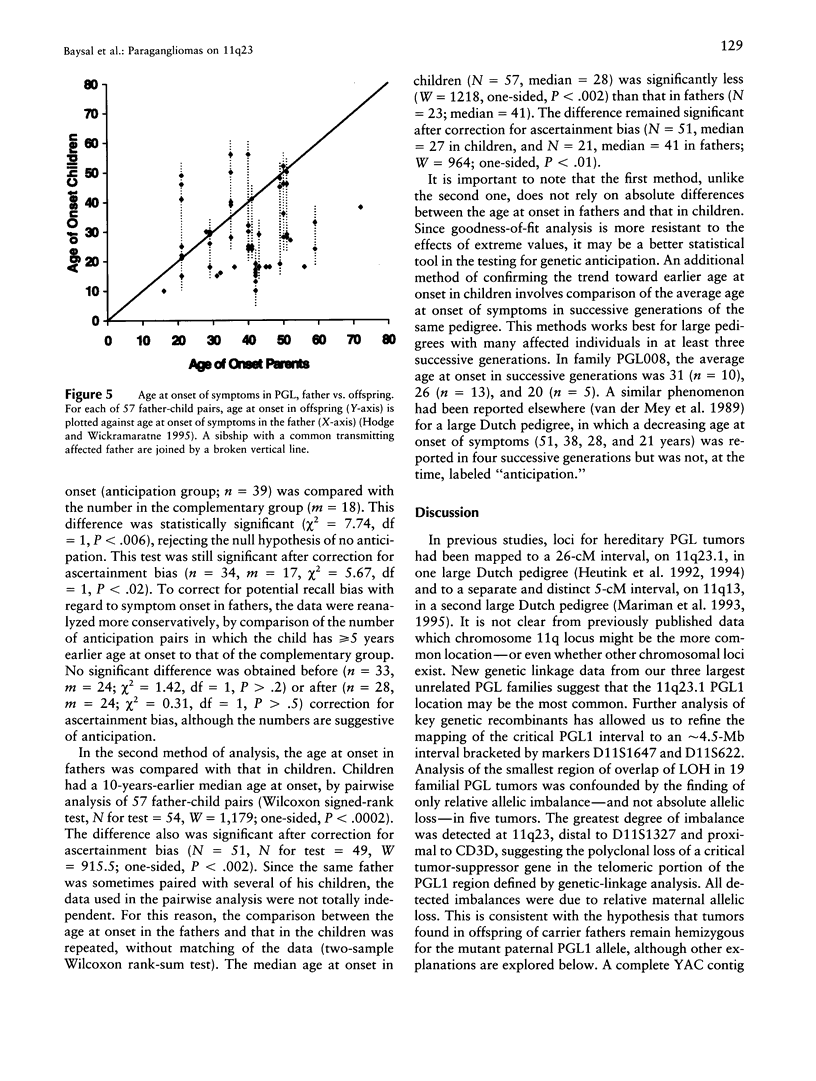
Hereditary nonchromaffin paragangliomas (PGL; glomus tumors; MIM 168000) are mostly benign, slow-growing tumors of the head and neck region, inherited from carrier fathers in an autosomal dominant fashion subject to genomic imprinting. Genetic linkage analysis in two large, unrelated Dutch families assigned PGL loci to two regions of chromosome 11, at 11q23 (PGL1) and 11q13.1 (PGL2). We ascertained a total of 11 North American PGL families and confirmed maternal imprinting (inactivation). In three of six families, linkage analysis provided evidence of linkage to the PGL1 locus at 11q23. Recombinants narrowed the critical region to an approximately 4.5-Mb interval flanked by markers D11S1647 and D11S622. Partial allelic loss of strictly maternal origin was detected in 5 of 19 tumors. The greatest degree of imbalance was detected at 11q23, distal to D11S1327 and proximal to CD3D. Age at onset of symptoms was significantly different between fathers and children (Wilcoxon rank-sum test, P < .002). Affected children had an earlier age at onset of symptoms in 39 of 57 father-child pairs (chi2 = 7.74, P < .006). However, a more conservative comparison of the number of pairs in which a child had > or = 5 years earlier age at onset (n = 33) vis-a-vis that of complementary pairs (n = 24) revealed no significant difference (chi2 = 1.42, P > .2). Whether these data represent genetic anticipation or ascertainment bias can be addressed only by analysis of a larger number of father-child pairs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashizawa T., Dunne C. J., Dubel J. R., Perryman M. B., Epstein H. F., Boerwinkle E., Hejtmancik J. F. Anticipation in myotonic dystrophy. I. Statistical verification based on clinical and haplotype findings. Neurology. 1992 Oct;42(10):1871–1877. doi: 10.1212/wnl.42.10.1871. [DOI] [PubMed] [Google Scholar]
- Barlow D. P. Gametic imprinting in mammals. Science. 1995 Dec 8;270(5242):1610–1613. doi: 10.1126/science.270.5242.1610. [DOI] [PubMed] [Google Scholar]
- Chaillet J. R., Vogt T. F., Beier D. R., Leder P. Parental-specific methylation of an imprinted transgene is established during gametogenesis and progressively changes during embryogenesis. Cell. 1991 Jul 12;66(1):77–83. doi: 10.1016/0092-8674(91)90140-t. [DOI] [PubMed] [Google Scholar]
- Chatkupt S., Antonowicz M., Johnson W. G. Parents do matter: genomic imprinting and parental sex effects in neurological disorders. J Neurol Sci. 1995 May;130(1):1–10. doi: 10.1016/0022-510x(94)00284-u. [DOI] [PubMed] [Google Scholar]
- Clark S. J., Harrison J., Frommer M. CpNpG methylation in mammalian cells. Nat Genet. 1995 May;10(1):20–27. doi: 10.1038/ng0595-20. [DOI] [PubMed] [Google Scholar]
- Coia L. R., Fazekas J. T., Farb S. N. Familial chemodectoma. Int J Radiat Oncol Biol Phys. 1981 Jul;7(7):949–952. doi: 10.1016/0360-3016(81)90014-6. [DOI] [PubMed] [Google Scholar]
- Devilee P., van Schothorst E. M., Bardoel A. F., Bonsing B., Kuipers-Dijkshoorn N., James M. R., Fleuren G., van der Mey A. G., Cornelisse C. J. Allelotype of head and neck paragangliomas: allelic imbalance is confined to the long arm of chromosome 11, the site of the predisposing locus PGL. Genes Chromosomes Cancer. 1994 Oct;11(2):71–78. doi: 10.1002/gcc.2870110202. [DOI] [PubMed] [Google Scholar]
- Dib C., Fauré S., Fizames C., Samson D., Drouot N., Vignal A., Millasseau P., Marc S., Hazan J., Seboun E. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature. 1996 Mar 14;380(6570):152–154. doi: 10.1038/380152a0. [DOI] [PubMed] [Google Scholar]
- Fick G. M., Johnson A. M., Gabow P. A. Is there evidence for anticipation in autosomal-dominant polycystic kidney disease? Kidney Int. 1994 Apr;45(4):1153–1162. doi: 10.1038/ki.1994.153. [DOI] [PubMed] [Google Scholar]
- Foulkes W. D., Campbell I. G., Stamp G. W., Trowsdale J. Loss of heterozygosity and amplification on chromosome 11q in human ovarian cancer. Br J Cancer. 1993 Feb;67(2):268–273. doi: 10.1038/bjc.1993.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grufferman S., Gillman M. W., Pasternak L. R., Peterson C. L., Young W. G., Jr Familial carotid body tumors: case report and epidemiologic review. Cancer. 1980 Nov 1;46(9):2116–2122. doi: 10.1002/1097-0142(19801101)46:9<2116::aid-cncr2820460934>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Hampton G. M., Mannermaa A., Winqvist R., Alavaikko M., Blanco G., Taskinen P. J., Kiviniemi H., Newsham I., Cavenee W. K., Evans G. A. Loss of heterozygosity in sporadic human breast carcinoma: a common region between 11q22 and 11q23.3. Cancer Res. 1994 Sep 1;54(17):4586–4589. [PubMed] [Google Scholar]
- Hampton G. M., Penny L. A., Baergen R. N., Larson A., Brewer C., Liao S., Busby-Earle R. M., Williams A. W., Steel C. M., Bird C. C. Loss of heterozygosity in cervical carcinoma: subchromosomal localization of a putative tumor-suppressor gene to chromosome 11q22-q24. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6953–6957. doi: 10.1073/pnas.91.15.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R. A., Larson A., Weiss J., Cavenee W. K., Hampton G. M., Arden K. C. A defined region of loss of heterozygosity at 11q23 in cutaneous malignant melanoma. Cancer Res. 1995 Jun 15;55(12):2494–2496. [PubMed] [Google Scholar]
- Heutink P., van Schothorst E. M., van der Mey A. G., Bardoel A., Breedveld G., Pertijs J., Sandkuijl L. A., van Ommen G. J., Cornelisse C. J., Oostra B. A. Further localization of the gene for hereditary paragangliomas and evidence for linkage in unrelated families. Eur J Hum Genet. 1994;2(3):148–158. doi: 10.1159/000472358. [DOI] [PubMed] [Google Scholar]
- Heutink P., van der Mey A. G., Sandkuijl L. A., van Gils A. P., Bardoel A., Breedveld G. J., van Vliet M., van Ommen G. J., Cornelisse C. J., Oostra B. A. A gene subject to genomic imprinting and responsible for hereditary paragangliomas maps to chromosome 11q23-qter. Hum Mol Genet. 1992 Apr;1(1):7–10. doi: 10.1093/hmg/1.1.7. [DOI] [PubMed] [Google Scholar]
- Hodge S. E., Wickramaratne P. Statistical pitfalls in detecting age-of-onset anticipation: the role of correlation in studying anticipation and detecting ascertainment bias. Psychiatr Genet. 1995 Spring;5(1):43–47. doi: 10.1097/00041444-199521000-00007. [DOI] [PubMed] [Google Scholar]
- Hoovers J. M., Kalikin L. M., Johnson L. A., Alders M., Redeker B., Law D. J., Bliek J., Steenman M., Benedict M., Wiegant J. Multiple genetic loci within 11p15 defined by Beckwith-Wiedemann syndrome rearrangement breakpoints and subchromosomal transferable fragments. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12456–12460. doi: 10.1073/pnas.92.26.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebos T. J., Slater R. M., Westerveld A. Inheritance of glomus tumours. Lancet. 1990 Mar 17;335(8690):660–660. doi: 10.1016/0140-6736(90)90446-c. [DOI] [PubMed] [Google Scholar]
- Höweler C. J., Busch H. F., Geraedts J. P., Niermeijer M. F., Staal A. Anticipation in myotonic dystrophy: fact or fiction? Brain. 1989 Jun;112(Pt 3):779–797. doi: 10.1093/brain/112.3.779. [DOI] [PubMed] [Google Scholar]
- Iizuka M., Sugiyama Y., Shiraishi M., Jones C., Sekiya T. Allelic losses in human chromosome 11 in lung cancers. Genes Chromosomes Cancer. 1995 May;13(1):40–46. doi: 10.1002/gcc.2870130107. [DOI] [PubMed] [Google Scholar]
- James M. R., Richard C. W., 3rd, Schott J. J., Yousry C., Clark K., Bell J., Terwilliger J. D., Hazan J., Dubay C., Vignal A. A radiation hybrid map of 506 STS markers spanning human chromosome 11. Nat Genet. 1994 Sep;8(1):70–76. doi: 10.1038/ng0994-70. [DOI] [PubMed] [Google Scholar]
- KROLL A. J., ALEXANDER B., COCHIOS F., PECHET L. HEREDITARY DEFICIENCIES OF CLOTTING FACTORS VII AND X ASSOCIATED WITH CAROTID-BODY TUMORS. N Engl J Med. 1964 Jan 2;270:6–13. doi: 10.1056/NEJM196401022700102. [DOI] [PubMed] [Google Scholar]
- Keldysh P. L., Dragani T. A., Fleischman E. W., Konstantinova L. N., Perevoschikov A. G., Pierotti M. A., Della Porta G., Kopnin B. P. 11q deletions in human colorectal carcinomas: cytogenetics and restriction fragment length polymorphism analysis. Genes Chromosomes Cancer. 1993 Jan;6(1):45–50. doi: 10.1002/gcc.2870060109. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Genetics of human cancer. Annu Rev Genet. 1986;20:231–251. doi: 10.1146/annurev.ge.20.120186.001311. [DOI] [PubMed] [Google Scholar]
- Koi M., Johnson L. A., Kalikin L. M., Little P. F., Nakamura Y., Feinberg A. P. Tumor cell growth arrest caused by subchromosomal transferable DNA fragments from chromosome 11. Science. 1993 Apr 16;260(5106):361–364. doi: 10.1126/science.8469989. [DOI] [PubMed] [Google Scholar]
- Mariman E. C., van Beersum S. E., Cremers C. W., Struycken P. M., Ropers H. H. Fine mapping of a putatively imprinted gene for familial non-chromaffin paragangliomas to chromosome 11q13.1: evidence for genetic heterogeneity. Hum Genet. 1995 Jan;95(1):56–62. doi: 10.1007/BF00225075. [DOI] [PubMed] [Google Scholar]
- Mariman E. C., van Beersum S. E., Cremers C. W., van Baars F. M., Ropers H. H. Analysis of a second family with hereditary non-chromaffin paragangliomas locates the underlying gene at the proximal region of chromosome 11q. Hum Genet. 1993 May;91(4):357–361. doi: 10.1007/BF00217356. [DOI] [PubMed] [Google Scholar]
- McCaffrey T. V., Meyer F. B., Michels V. V., Piepgras D. G., Marion M. S. Familial paragangliomas of the head and neck. Arch Otolaryngol Head Neck Surg. 1994 Nov;120(11):1211–1216. doi: 10.1001/archotol.1994.01880350023005. [DOI] [PubMed] [Google Scholar]
- Moulton T., Crenshaw T., Hao Y., Moosikasuwan J., Lin N., Dembitzer F., Hensle T., Weiss L., McMorrow L., Loew T. Epigenetic lesions at the H19 locus in Wilms' tumour patients. Nat Genet. 1994 Jul;7(3):440–447. doi: 10.1038/ng0794-440. [DOI] [PubMed] [Google Scholar]
- Negrini M., Rasio D., Hampton G. M., Sabbioni S., Rattan S., Carter S. L., Rosenberg A. L., Schwartz G. F., Shiloh Y., Cavenee W. K. Definition and refinement of chromosome 11 regions of loss of heterozygosity in breast cancer: identification of a new region at 11q23.3. Cancer Res. 1995 Jul 15;55(14):3003–3007. [PubMed] [Google Scholar]
- O'Connell J. R., Weeks D. E. The VITESSE algorithm for rapid exact multilocus linkage analysis via genotype set-recoding and fuzzy inheritance. Nat Genet. 1995 Dec;11(4):402–408. doi: 10.1038/ng1295-402. [DOI] [PubMed] [Google Scholar]
- Parry D. M., Li F. P., Strong L. C., Carney J. A., Schottenfeld D., Reimer R. R., Grufferman S. Carotid body tumors in humans: genetics and epidemiology. J Natl Cancer Inst. 1982 Apr;68(4):573–578. [PubMed] [Google Scholar]
- Reid L. H., West A., Gioeli D. G., Phillips K. K., Kelleher K. F., Araujo D., Stanbridge E. J., Dowdy S. F., Gerhard D. S., Weissman B. E. Localization of a tumor suppressor gene in 11p15.5 using the G401 Wilms' tumor assay. Hum Mol Genet. 1996 Feb;5(2):239–247. doi: 10.1093/hmg/5.2.239. [DOI] [PubMed] [Google Scholar]
- Sapienza C. Parental origin effects, genome imprinting, and sex-ratio distortion: double or nothing? Am J Hum Genet. 1994 Dec;55(6):1073–1075. [PMC free article] [PubMed] [Google Scholar]
- Selker E. U. Premeiotic instability of repeated sequences in Neurospora crassa. Annu Rev Genet. 1990;24:579–613. doi: 10.1146/annurev.ge.24.120190.003051. [DOI] [PubMed] [Google Scholar]
- Shaw M. E., Knowles M. A. Deletion mapping of chromosome 11 in carcinoma of the bladder. Genes Chromosomes Cancer. 1995 May;13(1):1–8. doi: 10.1002/gcc.2870130102. [DOI] [PubMed] [Google Scholar]
- Srivatsan E. S., Ying K. L., Seeger R. C. Deletion of chromosome 11 and of 14q sequences in neuroblastoma. Genes Chromosomes Cancer. 1993 May;7(1):32–37. doi: 10.1002/gcc.2870070106. [DOI] [PubMed] [Google Scholar]
- Steenman M. J., Rainier S., Dobry C. J., Grundy P., Horon I. L., Feinberg A. P. Loss of imprinting of IGF2 is linked to reduced expression and abnormal methylation of H19 in Wilms' tumour. Nat Genet. 1994 Jul;7(3):433–439. doi: 10.1038/ng0794-433. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. S., Nelson D. L., Zhang F., Pieretti M., Caskey C. T., Saxe D., Warren S. T. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992 Sep;1(6):397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. P., Gammack A. J., Stickland J. E., Mann G. J., MacKie R. M., Kefford R. F., McGee J. O. Loss of heterozygosity in malignant melanoma at loci on chromosome 11 and 17 implicated in the pathogenesis of other cancers. Genes Chromosomes Cancer. 1993 Jul;7(3):169–172. doi: 10.1002/gcc.2870070310. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- van der Mey A. G., Maaswinkel-Mooy P. D., Cornelisse C. J., Schmidt P. H., van de Kamp J. J. Genomic imprinting in hereditary glomus tumours: evidence for new genetic theory. Lancet. 1989 Dec 2;2(8675):1291–1294. doi: 10.1016/s0140-6736(89)91908-9. [DOI] [PubMed] [Google Scholar]


