Abstract
Ezrin is a member of the ERM (Ezrin-Radixin-Moesin) family of membrane-cytoskeletal linking proteins. ERM proteins are involved in a wide variety of cellular functions including cell motility, signal transduction, cell-cell interaction and cell-matrix recognition. A recent in situ hybridization study showed that the mRNA encoding ezrin is expressed in neurogenic regions of the mature brain including the subventricular zone (SVZ) and rostral migratory stream (RMS); however, the specific cell types expressing ezrin and their relationship to migrating and proliferating cells in these regions have not been characterized previously.
In this study, we used immunocytochemistry to perform double labeling with a variety of cell-type specific markers to characterize the expression of ezrin in the adult SVZ and RMS. Ezrin was expressed at high levels in both the SVZ and RMS where ezrin-immunopositive processes formed a trabecular network surrounding the proliferating and migrating cells. Ezrin-positive cells co-labeled with the glial makers S100β and GFAP (glial fibrillary acidic protein), but only minimally with the early neuronal markers β III tubulin and PSA-NCAM, indicating that ezrin was expressed primarily in the glial tube cells. Ezrin positive cells also expressed β-catenin, a membrane-complex protein previously implicated in the regulation of stem-cell proliferation and neuronal migration. Glial tube cells act as both precursors of, and a physical channel for, migrating neuroblasts. Bi-directional signals between glial tube cells and migrating neuroblasts have been shown to regulate the rates of both proliferation of the precursor cells and migration of the newly generated neuroblasts. Our finding that ezrin and β-catenin are both present at the cell membrane of the glial tube cells suggests that these proteins may be involved in those signaling processes.
Keywords: adult neurogenesis, olfactory bulb, ERM protein, beta- catenin, membrane complex proteins
Introduction
The SVZ is one of two regions in the adult brain that produce new neurons throughout the adult life span (reviewed in (Alvarez-Buylla and Garcia-Verdugo, 2002, Abrous et al., 2005)). Neuronal precursors destined for the olfactory bulb initially divide in the SVZ, and then migrate long distances, first tangentially through the RMS and then radially into the outer layers of the olfactory bulb (Luskin, 1993, Lois and Alvarez-Buylla, 1994). Stem cells located in the adult SVZ appear to include distinct pools of stem cells that give rise to granule and periglomerular neurons (Beech et al., 2004, Kohwi et al., 2005, Lemasson et al., 2005). Migrating cells in the SVZ and RMS are ensheathed by a network of astrocytic cells, sometimes referred to as glial tubes (Peretto et al., 1997). The cells comprising the glial tubes serve both as the precursors of, and a physical channel for, the migrating cells (Doetsch et al., 1999, Alvarez-Buylla and Garcia-Verdugo, 2002).
Ezrin is a member of the ERM family of membrane-cytoskeletal linking proteins (Gould et al., 1989, Takeuchi et al., 1994). ERM proteins are involved in a wide variety of cellular functions including cell motility, signal transduction, cell-cell interaction and cell-matrix recognition (Louvet-Vallee, 2000, Bretscher et al., 2002). Ezrin expression has been detected in peripheral astrocytic processes in the rat hippocampus and in primary cultured astrocytes (Derouiche and Frotscher, 2001). In addition, a recent in situ hybridization study showed ezrin mRNA expression in neurogenic regions of the mature brain, including the SVZ and RMS (Gimeno et al., 2004). However, the specific cell types expressing ezrin and their relationship to migrating and proliferating cells in these regions have not been characterized previously.
In this study, we used immunocytochemistry to perform double labeling with a variety of cell-type specific markers, including the glial markers, S100β and GFAP, and the early neuronal markers, β III tubulin (Peretto et al., 1997) and PSA-NCAM (Hu et al., 1996), to characterize the expression of ezrin in the adult SVZ and RMS. The thymidine analog bromodeoxyuridine (BrdU) was also used to label dividing cells to investigate the relationship of ezrin-expressing cells to the proliferating and migrating cells in the SVZ and RMS. We also investigated the expression of β-catenin, another membrane-cytoskeletal linking protein that has previously been implicated in the regulation of proliferation in both SVZ and other stem-cell populations (Chenn and Walsh, 2002, Chenn and Walsh, 2003, Gavard et al., 2004, He et al., 2004, Reya and Clevers, 2005).
We found that both ezrin and β-catenin were expressed primarily in glial tube cells. Bidirectional signals between glial tube cells and migrating neuroblasts have been shown to regulate the rates of both proliferation of the precursor cells and migration of the newly generated neuroblasts (Bolteus and Bordey, 2004, Liu et al., 2005). Our finding that ezrin and β-catenin are both present at the cell membrane of the glial tube cells suggests that these proteins may be involved in those signaling processes.
Materials and Methods
All procedures involving animals were performed in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Yale Animal Care and Use Committee.
BrdU Labeling and Tissue Preparation
Mice received a single intraperitoneal injection of BrdU (75 mg/kg) (Roche Diagnostics, Indianapolis, IN). Two hrs later, mice were deeply anesthetized with chloral hydrate and perfused transcardially with 4% paraformaldehyde. Brains were dissected, post-fixed for 48 hrs in 4% paraformaldehyde, cryoprotected in 30% sucrose and cut into serial 30-μm coronal or parasagittal sections on a freezing microtome.
Primary Antibodies and Immunofluorescence
Primary antibodies were purchased from BD Pharmingen (San Diego, CA), Cell Signaling Technology (Beverly, MA), Chemicon (Temecula, CA), Sigma-Aldrich (St. Louis, MO), and Upstate Cell Signaling (Lake Placid, NY). The sources of primary antibodies, specificities and the concentration used for each antibody are listed in table I. Secondary antibodies (Alexa-488 anti-mouse IgG (highly purified), Alexa-488 anti-mouse IgG1, Alexa-568 anti-rabbit IgG, Alexa-568 anti-mouse IgG2a, Alexa-568 anti-mouse IgM, and Alexa 633-anti-rat IgG) were purchased from Molecular Probes (Eugene, OR) and were used at a dilution of 1:2,000. For immunofluorescence microscopy, free floating sections were washed in potassium-phosphate buffered saline (KPBS), incubated in 2N HCl (for BrdU staining), blocked in KPBS + 0.3% Triton X-100 + 4% Normal Serum for one hr, and incubated in primary antibodies for 48 hrs at 4° C. After washes, secondary antibodies were added for 1 hr, and sections were washed and then incubated for 5 min in filtered 70% methanol with 0.7% Sudan Black B to quench autofluorescence. Co-localization of different markers was assessed at 40x and 63x in a z-series of images obtained in multi-track mode on a Zeiss LSM 510 or LSM 510 META laser scanning confocal microscope.
TABLE 1.
Primary antibodies: Sources, Specificities, and Dilutions.
| Primary antibody | Source, catalog #, specificity. | Dilution |
|---|---|---|
| 1. rabbit anti-ezrin | Source: Upstate Cell Signaling, # 07-130 Immunogen: Recombinant C-terminal human ezrin (amino acids 479-498). Specificity: human, mouse ezrin (no cross-reactivity to radixin or moesin). |
1:100 to 1:4,000 |
| 2. mouse monoclonal anti-ezrin |
Source: Sigma-Aldrich, Clone: 3C12. Isotype: mouse IgG1 Immunogen: carboxy-terminal section of recombinant human ezrin (amino acids 362-585). Specificity: human, monkey, bovine, kangaroo rat, hamster, mouse ezrin (no cross-reactivity to radixin or moesin). |
1:100 to 1:4,000 |
| 3.rat monoclonal anti- bromodeoxyuridine |
Source: Accurate Chemical, Clone: BU1/75 (ICR1) Isotype: rat IgG2a Immunogen: BrdU Specificity: BrdU in single stranded DNA, attached to a carrier protein or free BrdU. |
1:100 |
| 4. rabbit anti-S100β | Source: Sigma Aldrich, Cat. # S2644 Immunogen: S-100 from bovine brain Specificity: glial and ependymal cells in the brain, Schwann cells of the peripheral nervous system, skin melanocytes, Langerhans cells, and most melanocytic tumors. |
1:500 |
| 5. mouse monoclonal anti-GFAP |
Source: Sigma-Aldrich, Clone: G-A-5 Isotype: mouse IgG1 Immunogen: GFAP from pig spinal cord Specificity: human, pig, rat, mouse GFAP. Stains astrocytes, Bergmann glia cells and chondrocytes. |
1:500 |
| 6. mouse monoclonal anti-GFAP |
Source: Chemicon International, clone: GF12-24 Isotype: mouse IgG2a Specificity: bovine, human, rat, mouse GFAP. |
1:500 |
| 7. rabbit anti-βIII tubulin |
Source: Sigma-Aldrich, Cat. # T2200. Immunogen: synthetic peptide corresponding to amino acid residues 441-450 of human β-tubulin III (Ala446 to Ser446 substitution) with N-terminal added cysteine. Specificity: human, mouse, rat β-tubulin III. |
1:500 |
| 8. mouse monoclonal anti- PSA-NCAM |
Source: Source: Chemicon International, clone: 2-2B Isotype: mouse IgM Immunogen: Viable Meningococcus group B (strain 355) Specificity: reacts with alpha 2-8 linked neuraminic acid (NeuAc-alpha 2-8) with n > 10. |
1:250- 1:500 |
| 9. mouse monoclonal anti-β-catenin |
Source: BD Pharmingen, Cat. # 610153 Isotype: mouse IgG1 Immunogen: Recombinant C-terminal mouse β-catenin (amino acids 571-781). Specificity: human, dog, rat, mouse, chick β-catenin. |
1:500 |
Peptide competition
For peptide competition studies, antibodies were used at the lowest effective concentration as determined experimentally. Antibodies were pre-incubated overnight at 4° C. with a 16-fold (weight/weight) excess of recombinant C-terminal Ezrin (amino acids 300-585) or an equal amount of Bovine Serum Albumin. Following pre-incubation, antibodies were used to carry out immunohistochemical staining as described above.
Immunoprecipitation and Western Analysis of Ezrin from Cell Lysates
Cell lysates (from Cos7 cells) were prepared in RIPA buffer (150mM NaCl, 50mM Tris-HCl pH 7.4, 1% Triton) containing protease inhibitor cocktail (Roche). Lysates were first incubated with monoclonal or polyclonal Ezrin antiserum for 1 hr at 4° C. and subsequently with protein ASepharose. Immune complexes were washed 3 times with cold RIPA buffer and resuspended in 1x SDS loading dye. Samples were resolved using SDS-PAGE, transferred to nitrocellulose membranes, and blocked in 3% BSA in TBS-T + 0.05% Tween, and incubated overnight in ezrin antibodies (1/1000).
Image acquisition and processing
Images of singly labeled sections (figure 1) were acquired on a Zeiss Axioscope II microscope. Images of doubly labeled sections (figures 2-6) were acquired using a Zeiss LSM 510 Meta laser scanning confocal microscope after excitation with Ar/Kr and He lasers and analyzed using the accompanying software. All images were processed using Adobe PhotoShop 7.0 (Adobe Systems, San Jose, CA) and Corel Draw 12.0 (Corel, Ottawa, ON, Canada). Images were adjusted for contrast and brightness to equilibrate light levels. Images were cropped, resized, and rotated for purposes of presentation. Images of double-labeled tissue were adjusted to equalize staining intensity levels and background in the images. In no case was the content of images altered.
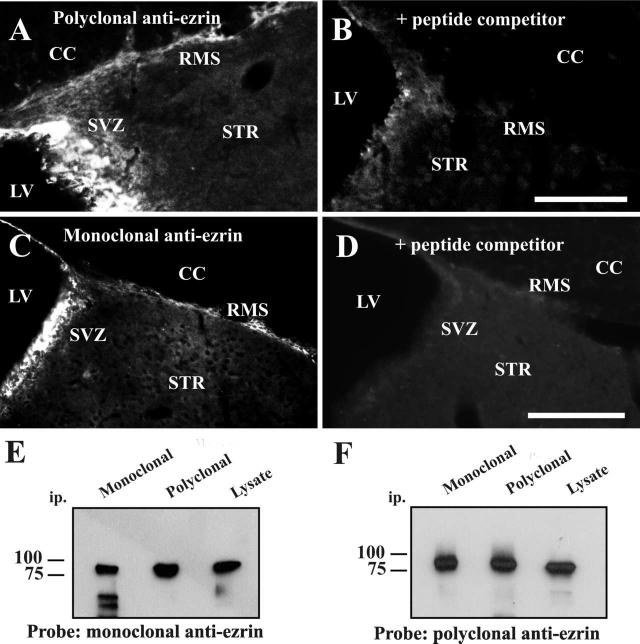
Figure 1.
Ezrin expression in the adult SVZ and RMS.
(A-D) Thirty micron sagittal sections from adult mouse brain were stained using two different anti-ezrin antibodies and detected by immunofluorescence: (A) rabbit polyclonal antibody made to a peptide corresponding to amino acids 479-498 of human ezrin (Upstate cell signaling), and (C) mouse monoclonal antibody which recognizes the carboxy-terminal section of recombinant human ezrin (amino acids 362-585; Sigma-Aldrich). Both antibodies showed a similar pattern of immunoreactivity with high expression in the SVZ and in cells lining the lateral ventricle, and lower expression in the RMS. The specificity of the observed staining was verified using a peptide competition assay, in which immunostaining by both polyclonal (B) and monoclonal (D) antibodies was blocked by pre-incubation with C-terminal ezrin . Scale bar in B= 100μm (applies to A,B). LV-lateral ventricle, SVZ- subventricular zone, RMS- rostral migratory stream, STR- striatum, CC- corpus callosum. Scale bar in D = 200μm (applies to C,D).
(E,F) Specificity of antibodies used: Immunoprecipitation from cell lysates using either monoclonal or polyclonal anti-ezrin, followed by western blotting revealed a single 80 kDa protein that was recognized by both monoclonal (E) and polyclonal (F) antibodies (lower molecular weight bands in the first lane of panel E correspond to heavy and light chains of the mouse IgG recognized by the secondary antibody).
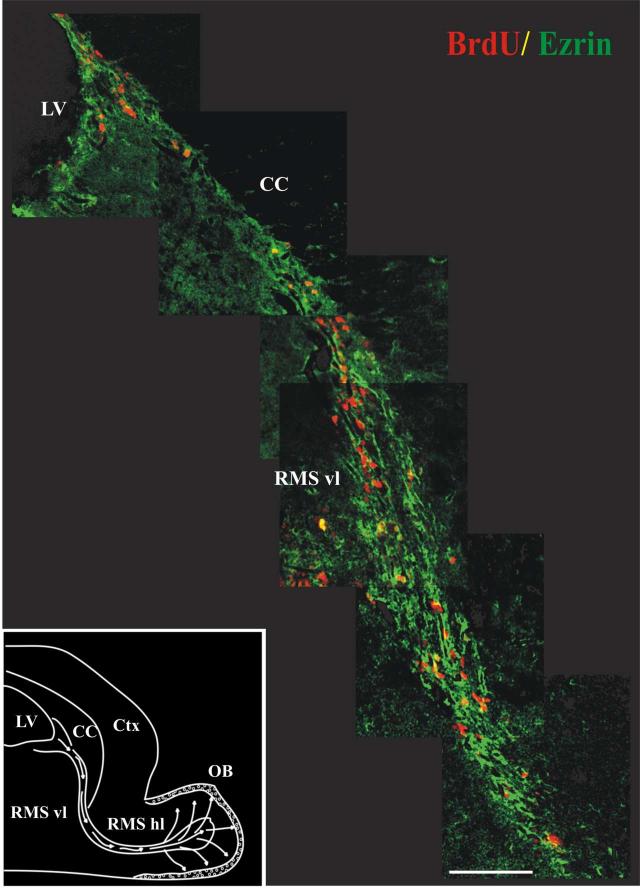
Figure 2.
Ezrin is expressed along the path of migrating cells in the RMS.
Montage of images starting from the SVZ and following the vertical limb of the RMS in a single thirty-micron sagittal section from an adult mouse sacrificed two hours after injection with BrdU. Sections were stained with antibodies to ezrin (green) and BrdU (red) and visualized by confocal microscopy after staining with fluorescently labeled secondary antibodies. As seen here, ezrin-immunopositive processes form a dense trabecular network surrounding the columns of BrdU-labeled migrating neuroblasts. These ezrin-immunopositive processes surround, but in most cases did not co-localize with, the BrdU-labeled neuroblasts. Inset: a schematic of the mouse forebrain illustrating the migratory pathway of SVZa derived neuroblasts en route to the olfactory bulb (adapted from (Coskun and Luskin, 2002). LV-Lateral Ventricle, CC- Corpus Callosum, Ctx - Cerebral Cortex, RMS vl - Vertical Limb of Rostral Migratory Stream, RMS hl - Horizontal Limb of Rostral Migratory Stream, OB - Olfactory Bulb. Scale bar = 100 μm.
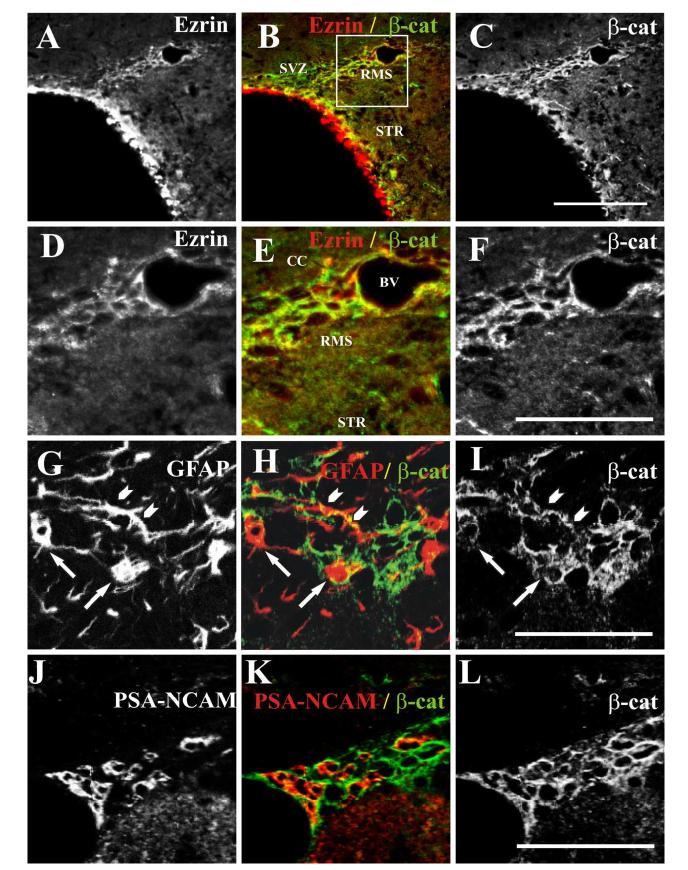
Figure 6.
Co-localization of β-catenin with ezrin and GFAP-immunopositive processes in the SVZ and RMS.
(A-C) Single confocal image showing co-localization of ezrin and β-catenin in the SVZ and RMS. Single confocal image from a thirty-micron coronal section from an adult mouse stained with rabbit polyclonal anti-ezrin (A) and mouse monoclonal anti-β-catenin (C). As seen here, ezrin immunoreactivity was present in the ependymal cells lining the lateral ventricles, but did not appear to co-localize with β-catenin in these cells. However, both proteins were present in the same cells in SVZ and RMS. The boxed region in panel B is shown at higher magnification in panels D-F. (A, D) ezrin, (B, E) merged image (ezrin, red, and β-catenin, green). (C, F) anti-β-catenin. Scale bar in C= 100 μm (applies to A-C). Scale bar in F= 50 μm (applies to D-F). β-catenin immunoreactivity also co-localized with staining for GFAP in the RMS. (G-I) Single confocal image of a thirty-micron coronal section from an adult mouse stained with antibodies to GFAP (G) and β-catenin (I) shows co-localization of the two proteins (merged image, H) in both cell bodies (arrows, G-I) and processes (arrowheads, G-I). Scale bar in I= 50 μm (applies to G-I). In contrast, this single confocal image of a section stained with antibodies to PSA-NCAM (J) and β-catenin (L), shows minimal overlap (merged image, K). Scale bar in L= 50 μm (applies to J-L).
Results
Ezrin is expressed in glial tubes in the adult SVZ and RMS
Ezrin expression in the adult SVZ and RMS was examined using two independent antibodies: a rabbit polyclonal antibody made to a peptide spanning amino acids 479-498 of human ezrin (Upstate Cell Signaling), and a mouse monoclonal antibody that recognizes the carboxy-terminal section of recombinant human ezrin (amino acids 362-585; Sigma-Aldrich). As shown in figure 1, both antibodies showed a similar pattern of immunoreactivity with high expression in the ependymal cells lining the lateral ventricle and lower expression in the SVZ and RMS. Staining with both antibodies was blocked by pre-incubation of the antisera with a peptide corresponding to the C-terminal fragment of ezrin (figure 1 A-D), and both antibodies recognized the same protein as demonstrated by immunoprecipitation and western blotting experiments (figure 1, E, F). Previous studies have shown that neither the mouse monoclonal (Bohling et al., 1996, Gronholm et al., 2005) nor the rabbit polyclonal (Yang and Hinds, 2003) antibodies cross-react with the related proteins radixin and moesin.
The thymidine analog bromodeoxyuridine (BrdU) was used to label dividing cells in order to determine the relationship between ezrin-expressing cells and the proliferating and migrating cells in the SVZ and rostral migratory stream (RMS). Animals were sacrificed 2 hrs after BrdU injection. As shown in figure 2, ezrin-immunoreactive processes are present in close proximity to BrdU-labeled (proliferating) cells in the SVZ and along the length of the RMS. Ezrin-immunopositive processes form a dense trabecular network surrounding the columns of BrdU-labeled migrating neuroblasts. In general, these processes surround, but do not co-localize with the BrdU-labeled neuroblasts. This appearance is consistent with the expression of ezrin in the astrocytic cells comprising the glial tubes in the SVZ and RMS. Occasional cells (<5% of total BrdU labeled cells) both in the SVZ and along the length of the RMS co-labeled with antibodies to both ezrin and BrdU (figure 3). These cells may represent glial tube cells undergoing mitosis.
Figure 3.
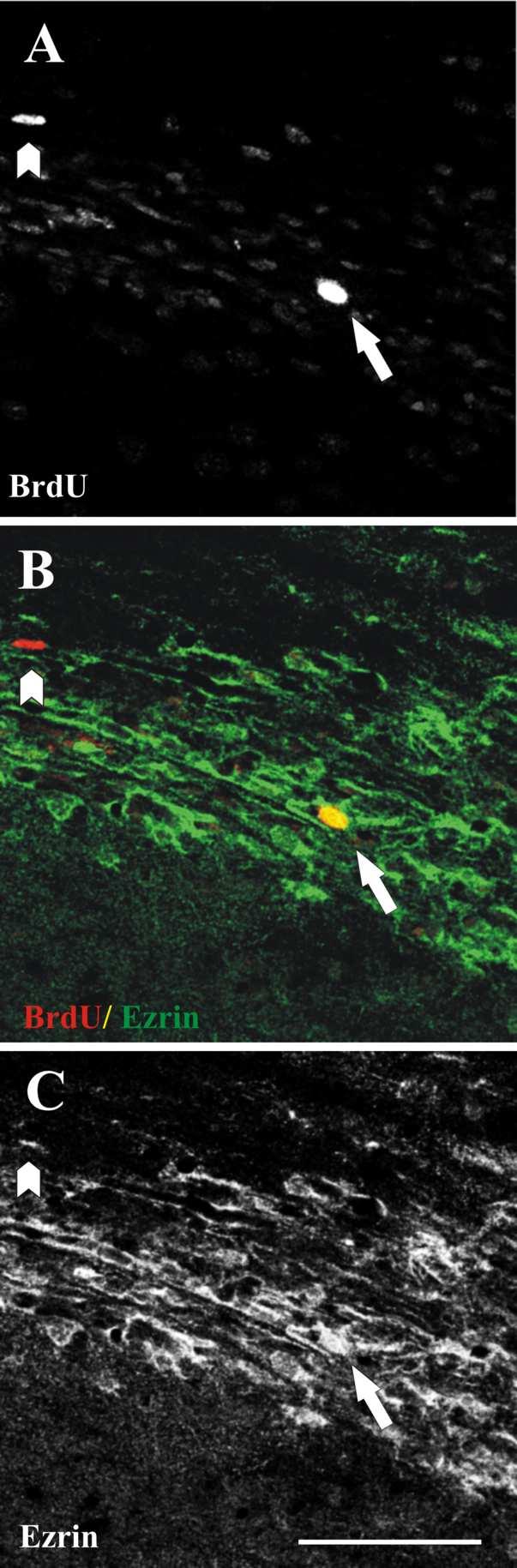
Co-localization of ezrin and BrdU in dividing cells in the RMS. Single confocal image from a thirty-micron sagittal section from an adult mouse demonstrating co-localization of ezrin and BrdU in one cell in the vertical limb of the RMS (arrows). (A) BrdU, (B) merged image (ezrin, green, and BrdU, red), (C) ezrin. A second BrdU+ cell that does not co-label with anti-ezrin is seen in the upper left (arrowhead). Scale bar in C= 50μm (applies to A-C).
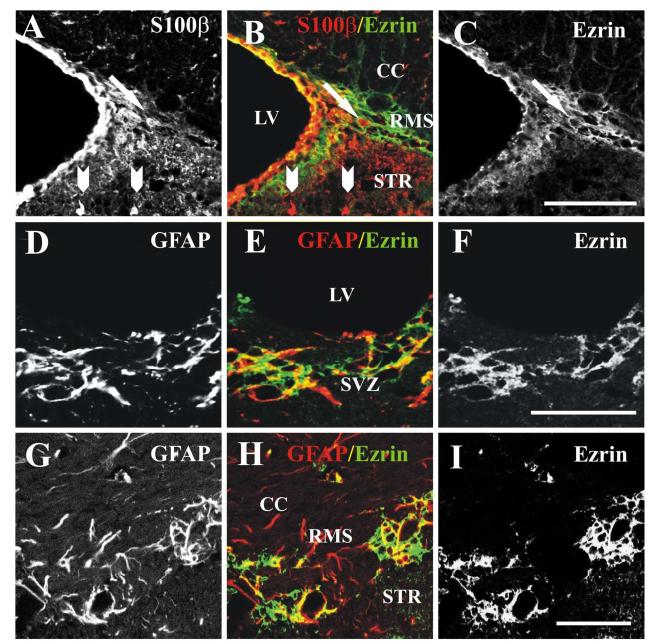
We next carried out double labeling experiments with ezrin and previously characterized glial markers including S100β and GFAP (Peretto et al., 1997). Ezrin staining partially co-localized with staining for both S100β (figure 4, A-C) and GFAP (figure 4, D-I), demonstrating that ezrin was expressed in glial tube cells. While antibodies to S100β primarily labeled cell bodies, anti-ezrin antibodies, like anti-GFAP, primarily labeled cell processes. However, Z-plane sectioning showed that S100β-labeled cell bodies in the RMS were clearly attached to ezrin-immunoreactive processes (arrow, figure 4, A-C). In contrast, S100β-positive cells outside the SVZ and RMS were not stained with ezrin antibodies (arrowheads, figure 4, A-C). Similarly, GFAP-immunopositive processes in both the SVZ (figure 4, D-F) and RMS (figure 4, G-I) were frequently co-labeled with antibodies to ezrin. However, staining for ezrin only partially co-localized with GFAP (figure 4, D-I), suggesting possible expression by other cell types as well.
Figure 4.
Co-localization of ezrin with glial markers in the SVZ and RMS.
(A-C) Single confocal image showing co-localization of ezrin with S100β in the SVZ and RMS in a thirty-micron sagittal section from an adult mouse. (A,) S100β, (B) merged image (ezrin, green, and S100β red), (C) ezrin. While antibodies to S100β primarily labeled cell bodies, anti-ezrin antibodies, primarily labeled cell processes. However, Z-plane sectioning showed that S100β-labeled cell bodies in the RMS (arrow, A-C) were clearly attached to ezrin-immunoreactive processes, while those outside the RMS did not stain with anti-ezrin antibodies (arrowheads at bottom of panel, A,B). Scale bar in C= 50 μm (applies to A-C). (D-F) Single confocal image showing co-localization of ezrin with GFAP in the SVZ in a thirty-micron sagittal section from an adult mouse. (A,) GFAP, (B) merged image (ezrin, green, and GFAP red), (C) ezrin. Scale bar in F= 50 μm (applies to D-F). (G-I) Single confocal image showing co-localization of ezrin with GFAP in the RMS in a thirty-micron coronal section from an adult mouse. (G) GFAP, (H) merged image (ezrin, green, and GFAP red), (I) ezrin. Scale bar in I= 50 μm (applies to G-I). LV-lateral ventricle, SVZ- subventricular zone, RMS- rostral migratory stream, STR- striatum, CC- corpus callosum.
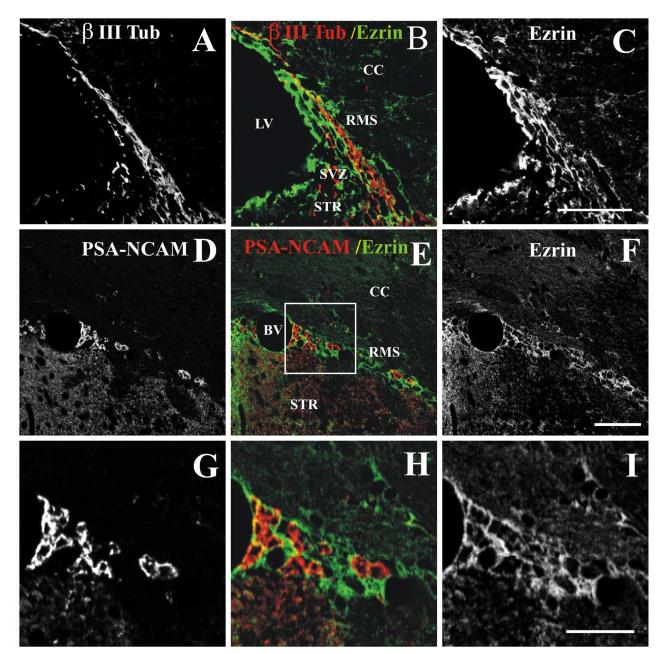
We then carried out double labeling experiments using antibodies to ezrin and the neuroblast markers βIII tubulin and PSA-NCAM to determine if ezrin is also expressed by migrating neuroblasts. As shown in figure 5 (A-C), in sagittal sections, cells arranged in long columns were stained with antibodies to either ezrin or βIII tubulin. Analyses of z-series of confocal images showed that ezrin was expressed primarily by cells surrounding the βIII tubulin-positive neuroblasts (figure 5, A-C). Coronal sections stained with antibodies to both PSA-NCAM and ezrin (figure 5, D-I), showed clusters of PSA-NCAM positive cells, surrounded by a network of ezrin-positive cells, with minimal overlap between the two staining patterns. This overlap (figure 5, B, E, H), which was seen primarily at the points of contact between the two cell types, may indicate that ezrin is also expressed at some level in the migrating neuroblasts, although the possibility of an artifact due to scatter cannot be excluded.
Figure 5.
Relationship between ezrin-immunoreactive processes and migrating neuroblasts.
(A-C). Single confocal image of a thirty-micron sagittal section from an adult mouse immunostained with antibodies to βIII tubulin, a marker of migrating neuroblasts (A), and ezrin (C) reveals a stream of immature precursors exiting the SVZ, surrounded by ezrin-immunoreactive processes , with minimal overlap between the two staining patterns (merged image, B). Scale bar in C= 50 μm (applies to A-C). (D-I) Single confocal image of a thirty-micron coronal section from an adult mouse immunostained with antibodies to PSA-NCAM (D), and Ezrin (F) shows a similar pattern of immunoreactivity, again demonstrating that ezrin is expressed primarily in the cells comprising the glial tube (merged image, E). The boxed area in panel E is shown at higher magnification in panels G-I. PSA-NCAM (G), (H) merged image, and Ezrin (I). Scale bar in F= 50 μm (applies to D-F). Scale bar in I= 25 μm (applies to G-I). LV-lateral ventricle, SVZ- subventricular zone, RMS- rostral migratory stream, STR- striatum, CC- corpus callosum, BV- blood vessel.
β-catenin is also expressed in glial tube cells
Next, we carried out double labeling studies with antibodies to ezrin and another membrane-cytoskeletal linking protein, β-catenin, that has previously been implicated in the regulation of proliferation and migration of neuronal precursors in the SVZ (Chenn and Walsh, 2002, Chenn and Walsh, 2003). Co-localization between ezrin and β-catenin was determined using the rabbit polyclonal anti-ezrin antibody and a mouse monoclonal antibody that recognizes amino acids 571-781 of the mouse β-catenin protein (figure 6A-F). As in figure 1, high levels of ezrin immunoreactivity were observed in the ependymal cells lining the lateral ventricles; however, this staining did not co-localize with staining for β-catenin. In contrast, both antisera stained the same cells in SVZ and RMS and staining was generally confined to the outer cell edges, consistent with their localization at the cell membrane (figure 6, A-F). In coronal sections through the RMS, β-catenin-immunoreactivity showed a pattern of staining highly similar to that of ezrin, including co-localization with staining for GFAP (figure 6, G-I), and minimal overlap with PSA-NCAM (figure 6, J-L). As with staining for ezrin, this overlap was seen primarily at the points of contact between the two cell types.
Discussion
In the present study, we used immunocytochemistry to perform double labelings with a variety of cell type specific markers to characterize the expression of ezrin in the adult SVZ and RMS. Ezrin immunoreactivity is present at high levels in both the SVZ and RMS, where ezrin-immunopositive processes form a trabecular network surrounding the proliferating and migrating cells. Ezrin-positive cells co-labeled extensively with the glial makers S100β and GFAP, but only minimally with the early neuronal marker β III tubulin and PSA-NCAM. This is consistent with a previous in vitro study showing that ezrin is expressed in astrocytic, but not neuronal cells derived from embryonic mouse neurospheres (Gronholm et al., 2005). Occasional cells were also seen which double-labeled with antibodies to ezrin and BrdU in both the SVZ and RMS. These cells appear to be glial tube cells undergoing mitosis.
Ezrin-immunopositive cells in the SVZ and RMS were also positive for β-catenin. β-catenin, in association with the transcription factor LEF1 (Porfiri et al., 1997), is a key downstream mediator of the wnt signaling pathway (Logan and Nusse, 2004). Wnt signaling inhibits neural differentiation of embryonic stem cells by up-regulating the expression bone morphogenetic proteins (BMPs) (Haegele et al., 2003). Endogenous β-catenin is highly expressed in embryonic neural precursors, and transgenic mice that express high levels of a stabilized β-catenin transgene develop enlarged brains with expanded precursor populations (Chenn and Walsh, 2002). Adult mice expressing the same β-catenin transgene develop enlarged forebrains with expanded subventricular zones (Chenn and Walsh, 2003). These results suggest that nuclear β-catenin can promote cell proliferation and inhibit differentiation of stem cells in the adult SVZ. In other cell types, ezrin forms a complex with Ecadherin and β-catenin that appears to regulate both cell-cell and cell-matrix recognition (Hiscox and Jiang, 1999). Disruption of this complex by tyrosine phosphorylation of ezrin leads to loss of cell-cell adhesion and increased invasiveness. These observations suggest that sequestration of β-catenin in membrane-associated complexes can serve to regulate the rate of cell division. However, which, if any, of the classic cadherins serves as a binding partner of β-catenin in the glial tube cells is currently unknown.
Ezrin was also highly expressed in the ependymal cells lining the lateral ventricles, however these cells did not co-label with antibodies to β-catenin. Ependymal cells secrete noggin (Lim et al., 2000), an inhibitor of BMP signaling, and thus promote neuronal differentiation of progenitor cells in the SVZ. This process is thought to be an important part of maintaining a neurogenic niche within the adult SVZ (Lim et al., 2000, Haegele et al., 2003, Peretto et al., 2004). In addition, beating of ependymal cilia has been proposed to play an important role in the creation of concentration gradients of CSF guidance molecules, and thus contribute vectorial information required for the directional migration of neuroblasts (Sawamoto et al., 2006). Ezrin is also expressed in other ciliated cell types including ciliated airway epithelia, where it is required for the apical localization of a number of proteins including EBP50 and the beta2 adrenergic receptor (Huang et al., 2003); however, the role, if any, of ezrin in the function of ependymal cilia has not yet been investigated.
Previous studies have shown that both the rate of proliferation of SVZ astrocytes and the rate of migration of newly generated neuroblasts are regulated by bi-directional signaling between these two cell types (Conover et al., 2000, Bolteus and Bordey, 2004, Liu et al., 2005). Notably, the only areas in which co-localization was observed between ezrin or β-catenin staining and the neuronal markers PSA-NCAM and beta tubulin were at the points of contact between the two cell types.
Signaling molecules that regulate both cell proliferation and migration do so, in large part, via their effects on the cytoskeleton (reviewed in (Beech, 2006)). Ezrin and β-catenin are both known to act as membrane-cytoskeletal cross-linking proteins. As outlined above, previous studies have suggested that β-catenin is a key regulator of cell proliferation in the SVZ and that sequestration of β-catenin with ezrin in membrane-associated complexes may be one way in which its activity is regulated. Our finding that ezrin is expressed in both β-catenin-expressing glial cells (which are the precursors of the migrating neuroblasts) and ependymal cells (which have been shown to promote neuronal differentiation) suggests that it may play a role in maintaining a neurogenic niche in the adult SVZ and RMS.
Acknowledgements
We thank Janine Leffert and Mary Morgan Taylor for their excellent technical assistance.
Comprehensive list of abbreviations
- BrdU
Bromodeoxyuridine
- ERM
Ezrin-Radixin-Moesin
- GFAP
Glial fibrillary acidic protein
- PSA-NCAM
Polysialylated form of neural cell adhesion molecule1
- RMS
Rostral migratory stream
- SZV
Subventricular zone
Literature Cited
- Abrous DN, Koehl M, Le Moal M. Adult Neurogenesis: From Precursors to Network and Physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech RD. Membrane-cytoskeletal interactions in the regulation of neuronal migration in the adult brain. In: Greer EV, editor. Progress in Stem Cell Research. Nova Science Publishers; Hauppauge, NY: 2006. [Google Scholar]
- Beech RD, Cleary MA, Treloar HB, Eisch AJ, Harrist AV, Zhong W, Greer CA, Duman RS, Picciotto MR. Nestin promoter/enhancer directs transgene expression to precursors of adult generated periglomerular neurons. J Comp Neurol. 2004;475:128–141. doi: 10.1002/cne.20179. [DOI] [PubMed] [Google Scholar]
- Bohling T, Turunen O, Jaaskelainen J, Carpen O, Sainio M, Wahlstrom T, Vaheri A, Haltia M. Ezrin expression in stromal cells of capillary hemangioblastoma. An immunohistochemical survey of brain tumors. Am J Pathol. 1996;148:367–373. [PMC free article] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb Cortex. 2003;13:599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- Coskun V, Luskin MB. Intrinsic and extrinsic regulation of the proliferation and differentiation of cells in the rodent rostral migratory stream. J Neurosci Res. 2002;69:795–802. doi: 10.1002/jnr.10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouiche A, Frotscher M. Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia. 2001;36:330–341. doi: 10.1002/glia.1120. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Gavard J, Marthiens V, Monnet C, Lambert M, Mege RM. N-cadherin activation substitutes for the cell contact control in cell cycle arrest and myogenic differentiation: involvement of p120 and beta-catenin. J Biol Chem. 2004;279:36795–36802. doi: 10.1074/jbc.M401705200. [DOI] [PubMed] [Google Scholar]
- Gimeno L, Corradi A, Cobos I, Consalez GG, Martinez S. Ezrin gene, coding for a membrane-cytoskeleton linker protein, is regionally expressed in the developing mouse neuroepithelium. Gene Expr Patterns. 2004;4:749–754. doi: 10.1016/j.modgep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Gould KL, Bretscher A, Esch FS, Hunter T. cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J. 1989;8:4133–4142. doi: 10.1002/j.1460-2075.1989.tb08598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronholm M, Teesalu T, Tyynela J, Piltti K, Bohling T, Wartiovaara K, Vaheri A, Carpen O. Characterization of the NF2 protein merlin and the ERM protein ezrin in human, rat, and mouse central nervous system. Mol Cell Neurosci. 2005;28:683–693. doi: 10.1016/j.mcn.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Haegele L, Ingold B, Naumann H, Tabatabai G, Ledermann B, Brandner S. Wnt signalling inhibits neural differentiation of embryonic stem cells by controlling bone morphogenetic protein expression. Mol Cell Neurosci. 2003;24:696–708. doi: 10.1016/s1044-7431(03)00232-x. [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Hiscox S, Jiang WG. Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci. 1999;112(Pt 18):3081–3090. doi: 10.1242/jcs.112.18.3081. [DOI] [PubMed] [Google Scholar]
- Hu H, Tomasiewicz H, Magnuson T, Rutishauser U. The role of polysialic acid in migration of olfactory bulb interneuron precursors in the subventricular zone. Neuron. 1996;16:735–743. doi: 10.1016/s0896-6273(00)80094-x. [DOI] [PubMed] [Google Scholar]
- Huang T, You Y, Spoor MS, Richer EJ, Kudva VV, Paige RC, Seiler MP, Liebler JM, Zabner J, Plopper CG, Brody SL. Foxj1 is required for apical localization of ezrin in airway epithelial cells. J Cell Sci. 2003;116:4935–4945. doi: 10.1242/jcs.00830. [DOI] [PubMed] [Google Scholar]
- Kohwi M, Osumi N, Rubenstein JLR, Alvarez-Buylla A. Pax6 Is Required for Making Specific Subpopulations of Granule and Periglomerular Neurons in the Olfactory Bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasson M, Saghatelyan A, Olivo-Marin J-C, Lledo P-M. Neonatal and Adult Neurogenesis Provide Two Distinct Populations of Newborn Neurons to the Mouse Olfactory Bulb. J Neurosci. 2005;25:6816–6825. doi: 10.1523/JNEUROSCI.1114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Louvet-Vallee S. ERM proteins: from cellular architecture to cell signaling. Biol Cell. 2000;92:305–316. doi: 10.1016/s0248-4900(00)01078-9. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Peretto P, Dati C, De Marchis S, Kim HH, Ukhanova M, Fasolo A, Margolis FL. Expression of the secreted factors noggin and bone morphogenetic proteins in the subependymal layer and olfactory bulb of the adult mouse brain. Neuroscience. 2004;128:685–696. doi: 10.1016/j.neuroscience.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Peretto P, Merighi A, Fasolo A, Bonfanti L. Glial tubes in the rostral migratory stream of the adult rat. Brain Res Bull. 1997;42:9–21. doi: 10.1016/s0361-9230(96)00116-5. [DOI] [PubMed] [Google Scholar]
- Porfiri E, Rubinfeld B, Albert I, Hovanes K, Waterman M, Polakis P. Induction of a beta-catenin-LEF-1 complex by wnt-1 and transforming mutants of beta-catenin. Oncogene. 1997;15:2833–2839. doi: 10.1038/sj.onc.1201462. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, Tessier-Lavigne M, Okano H, Alvarez-Buylla A. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Kawashima A, Nagafuchi A, Tsukita S. Structural diversity of band 4.1 superfamily members. J Cell Sci. 1994;107:1921–1928. doi: 10.1242/jcs.107.7.1921. [DOI] [PubMed] [Google Scholar]
- Yang HS, Hinds PW. Increased ezrin expression and activation by CDK5 coincident with acquisition of the senescent phenotype. Mol Cell. 2003;11:1163–1176. doi: 10.1016/s1097-2765(03)00135-7. [DOI] [PubMed] [Google Scholar]