Abstract
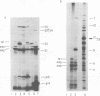
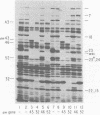
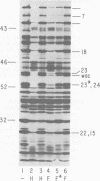
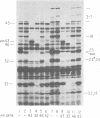
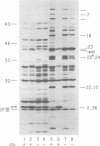
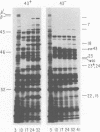
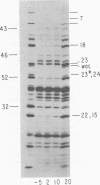
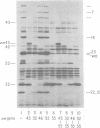
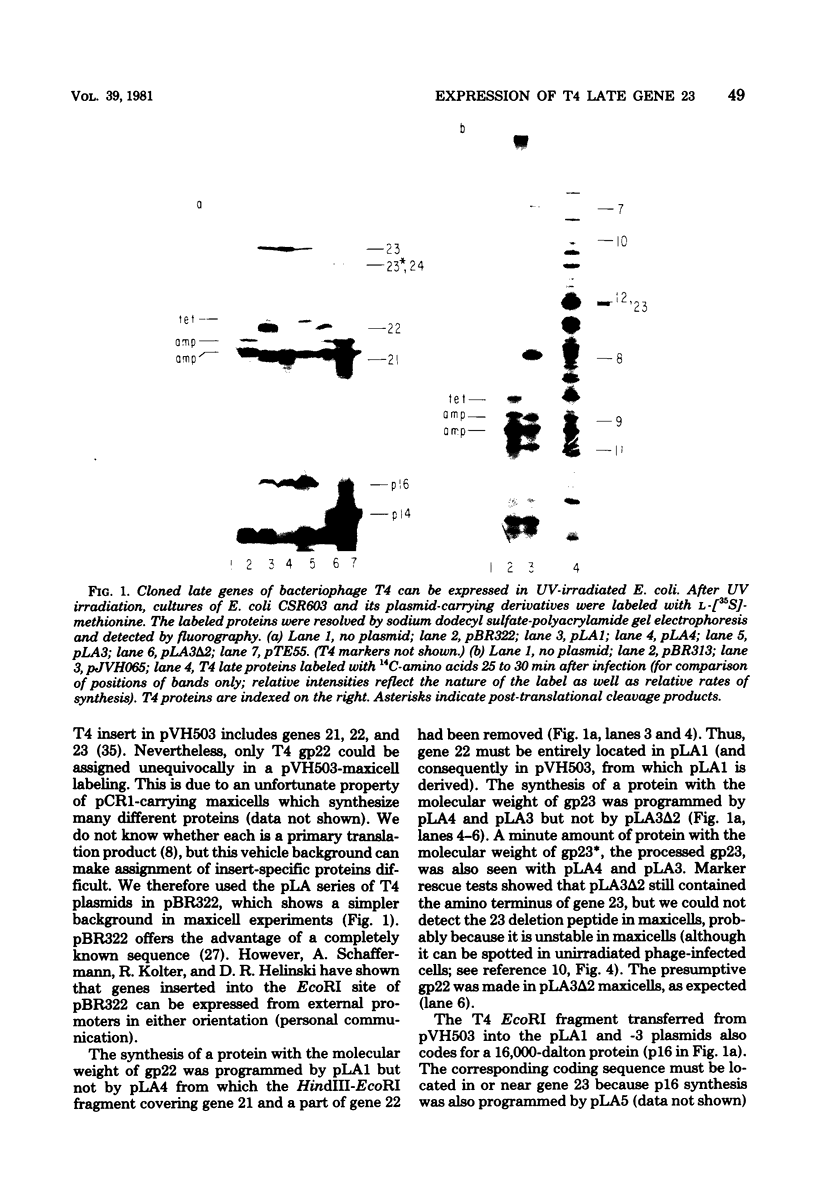
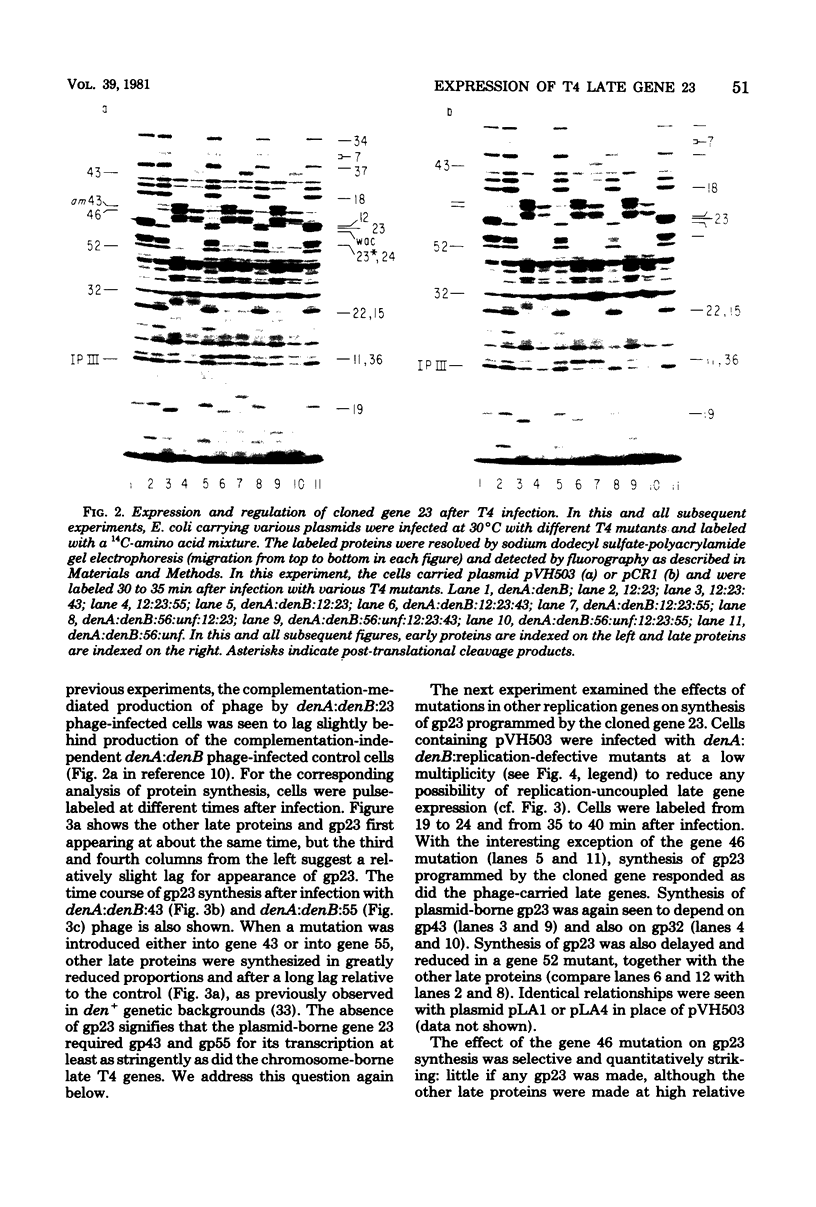
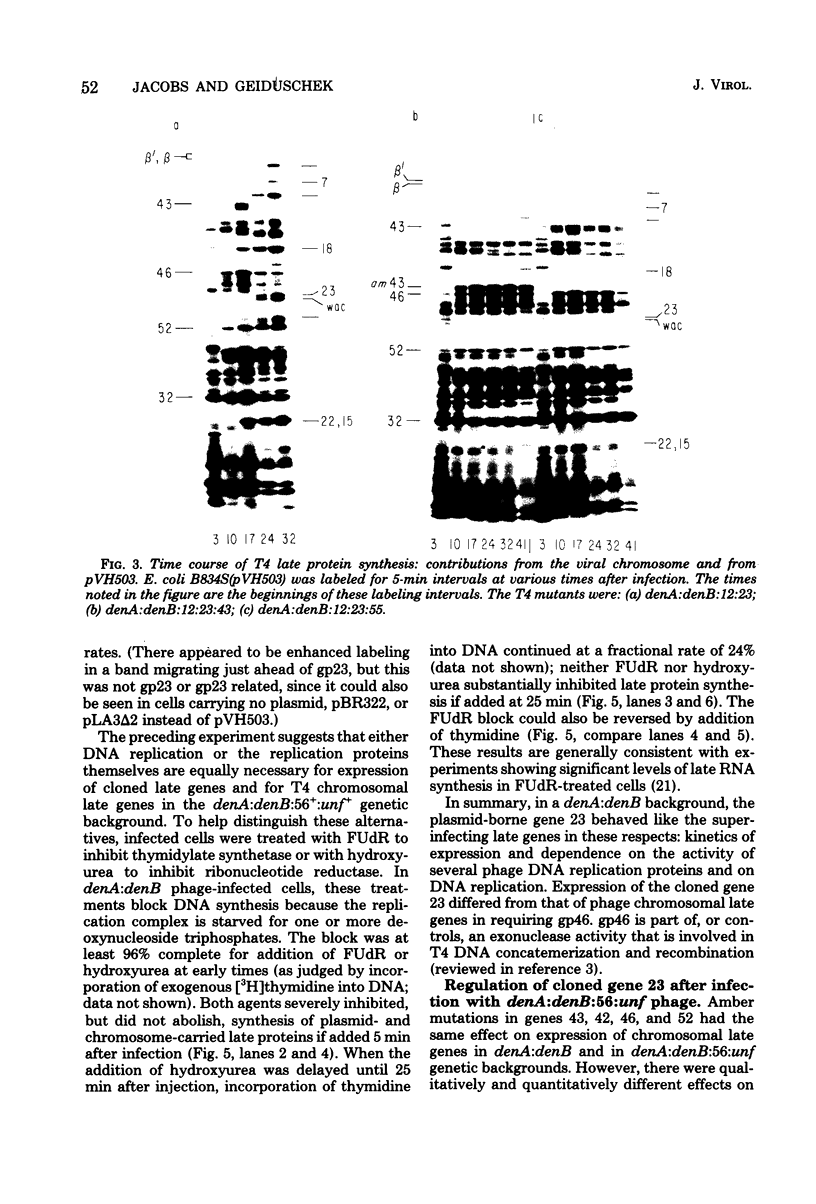
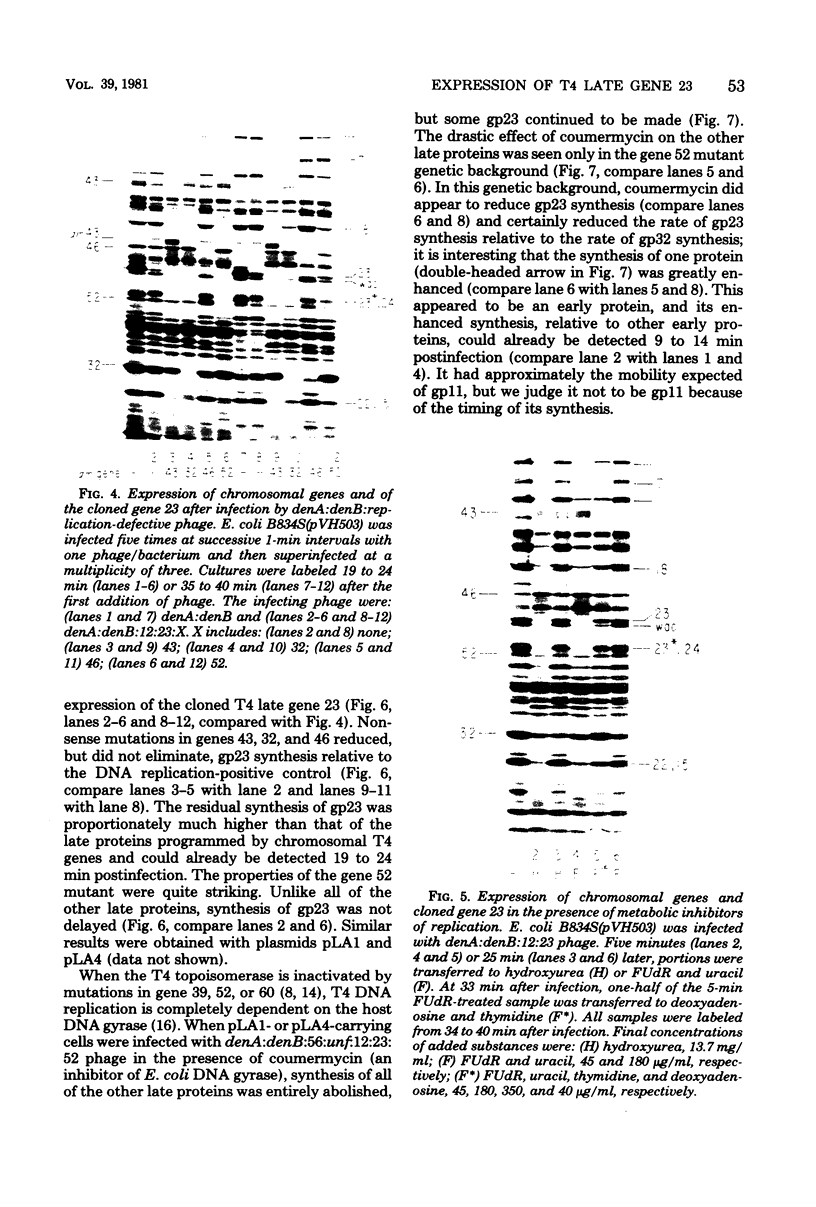
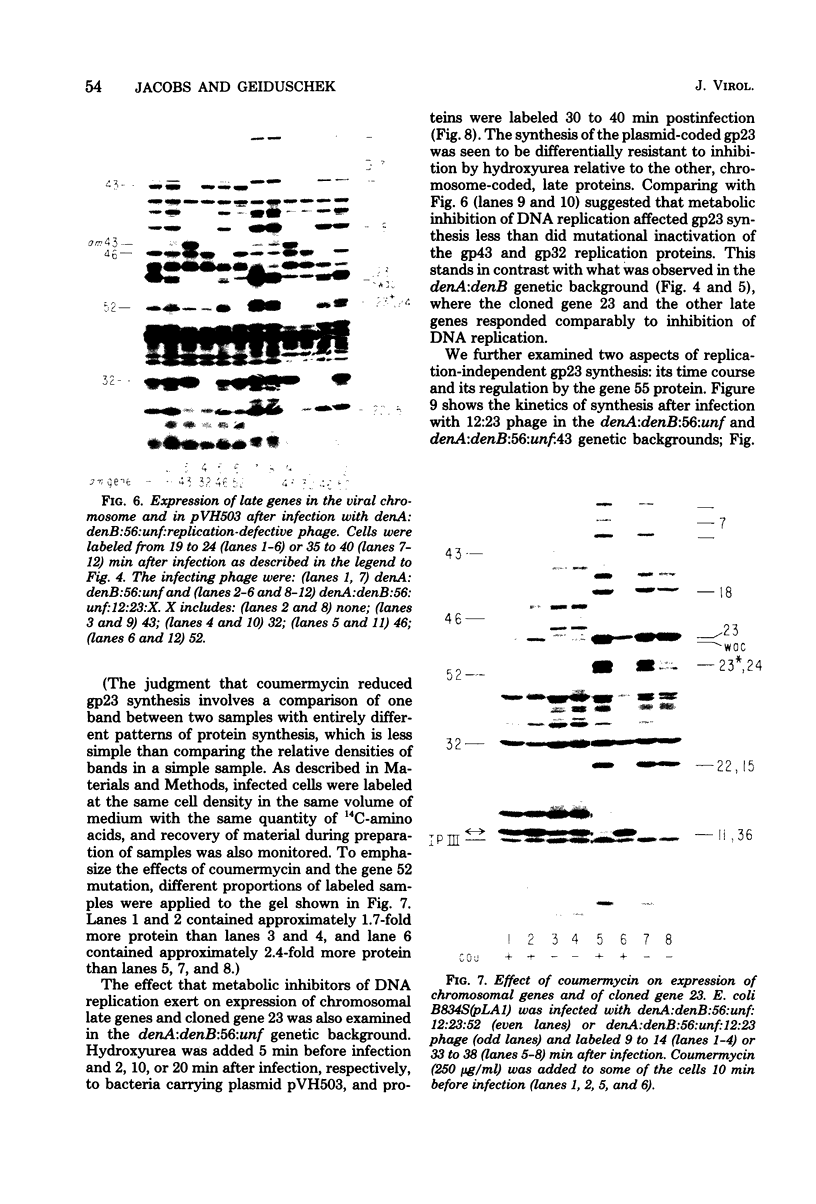
The parameters governing the activity of the cloned T4 gene 23, which codes for the major T4 head protein, were analyzed. Suppressor-negative bacteria carrying wild-type T4 gene 23 cloned into plasmid pCR1 or pBR322 were infected with T4 gene 23 amber phage also carrying mutations in the following genes: (i) denA and denB (to prevent breakdown of plasmid DNA after infection) and (ii) denA, denB, and, in addition, 56 (to generate newly replicated DNA containing dCMP) and alc/unf (because mutations in this last gene allow late genes to be expressed in cytosine-containing T4 DNA). Bacteria infected with these phage were labeled with 14C-amino acids at various times after infection, and the labeled proteins were separated by one-dimensional gel electrophoresis so that the synthesis of plasmid-coded gp23 could be compared with the synthesis of other, chromosome-coded T4 late proteins. We analyzed the effects of additional mutations that inactivate DNA replication proteins (genes 32 and 43), an RNA polymerase-binding protein (gene 55), type II topoisomerase (gene 52), and an exonuclease function involved in recombination (gene 46) on the synthesis of plasmid-coded gp23 in relation to chromosome-coded T4 late proteins. In the denA:denB:56:alc/unf genetic background, the phage chromosome-borne late genes followed the same regulatory rules (with respect to DNA replication and gp55 action) as in the denA:denB genetic background. The plasmid-carried gene 23 was also under gp55 control, but was less sensitive than the chromosomal late genes to perturbations of DNA replication. Synthesis of plasmid-coded gp23 was greatly inhibited when both the type II T4 topoisomerase and the host's DNA gyrase are inactivated. Synthesis of gp23 was also substantially affected by a mutation in gene 46, but less strongly than in the denA:denB genetic background. These observations are interpreted as follows. The plasmid-borne T4 gene 23 is primarily expressed from a late promoter. Expression of gene 23 from this late promoter responds to an activation event which involves some structural alteration of DNA. In these respects, the requirements for expressing the plasmid-borne gene 23 and chromosomal late genes are very similar (although in the denA:denB:56:alc/unf genetic background, there are significant quantitative differences). For the plasmid-borne gene 23, activation involves the T4 gp46, a protein which is required for DNA recombination. However, for the reasons presented in the accompanying paper (Jacobs et al., J. Virol. 39:31-45, 1981), we conclude that the activation of gene 23 does not require a complete breakage-reunion event which transposes that gene to a later promoter on the phage chromosome. Ways in which gp46 may actually be involved in late promoter activation on the plasmid are discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Doermann A. H. Molecular and genetic recombination of bacteriophage T4. Annu Rev Genet. 1975;9:213–244. doi: 10.1146/annurev.ge.09.120175.001241. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Lehman I. R. Branched DNA molecules: intermediates in T4 recombination. J Mol Biol. 1971 Aug 28;60(1):131–149. doi: 10.1016/0022-2836(71)90453-0. [DOI] [PubMed] [Google Scholar]
- Campbell J. L., Richardson C. C., Studier F. W. Genetic recombination and complementation between bacteriophage T7 and cloned fragments of T7 DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2276–2280. doi: 10.1073/pnas.75.5.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. E., Mattson T., Kozinski A. W. Origins of phage T4 DNA replication as revealed by hybridization to cloned genes. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6137–6141. doi: 10.1073/pnas.76.12.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlett N. V., Berger H. Mutations altering genetic recombination and repair of DNA in bacteriophage T4. Virology. 1975 Feb;63(2):539–567. doi: 10.1016/0042-6822(75)90326-8. [DOI] [PubMed] [Google Scholar]
- Huang W. M. Positive regulation of T-even-phage DNA replication by the DNA-delay protein of gene 39. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):495–499. doi: 10.1101/sqb.1979.043.01.055. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Applebaum B. Proteins synthesized in minicells containing plasmid ColE1 and its mutants. J Bacteriol. 1978 Mar;133(3):1444–1451. doi: 10.1128/jb.133.3.1444-1451.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs K. A., Albright L. M., Shibata D. K., Geiduschek E. P. Genetic complementation by cloned bacteriophage T4 late genes. J Virol. 1981 Jul;39(1):31–45. doi: 10.1128/jvi.39.1.31-45.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisch H. M., Bolle A., Epstein R. H. Regulation of the synthesis of bacteriophage T4 gene 32 protein. J Mol Biol. 1974 Sep 5;88(1):89–104. doi: 10.1016/0022-2836(74)90296-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. T4 DNA topoisomerase: a new ATP-dependent enzyme essential for initiation of T4 bacteriophage DNA replication. Nature. 1979 Oct 11;281(5731):456–461. doi: 10.1038/281456a0. [DOI] [PubMed] [Google Scholar]
- Mattson T., Van Houwe G., Bolle A., Selzer G., Epstein R. Genetic identification of cloned fragments of bacteriophage T4 DNA and complementation by some clones containing early T4 genes. Mol Gen Genet. 1977 Sep 9;154(3):319–326. doi: 10.1007/BF00571289. [DOI] [PubMed] [Google Scholar]
- McCarthy D. Gyrase-dependent initiation of bacteriophage T4 DNA replication: interactions of Escherichia coli gyrase with novobiocin, coumermycin and phage DNA-delay gene products. J Mol Biol. 1979 Jan 25;127(3):265–283. doi: 10.1016/0022-2836(79)90329-2. [DOI] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M., Lehman I. R. Initiation of general recombination catalyzed in vitro by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2615–2619. doi: 10.1073/pnas.76.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D., Kutter E. M., Guttman B. S. Synthesis of T4 DNA and bacteriophage in the absence of dCMP hydroxymethylase. J Virol. 1978 Oct;28(1):262–269. doi: 10.1128/jvi.28.1.262-269.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G., Luder A., Garcia G., Dannenberg R., Bock S. In vivo interactions of genes and proteins in DNA replication and recombination of phage T4. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):501–515. doi: 10.1101/sqb.1979.043.01.056. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., DasGupta C., Cunningham R. P., Radding C. M. Purified Escherichia coli recA protein catalyzes homologous pairing of superhelical DNA and single-stranded fragments. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1638–1642. doi: 10.1073/pnas.76.4.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe M. K., Onorato L. Kinetic factors and form determination of the head of bacteriophage T4. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4165–4169. doi: 10.1073/pnas.75.9.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L., Gold L., Kutter E. A gene of bacteriophage T4 whose product prevents true late transcription on cytosine-containing T4 DNA. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3098–3102. doi: 10.1073/pnas.73.9.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler G. L., King G. J., Huang W. M. T4 DNA-delay proteins, required for specific DNA replication, form a complex that has ATP-dependent DNA topoisomerase activity. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3737–3741. doi: 10.1073/pnas.76.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., Chiu C. S., Wovcha M. G., Greenberg G. R. Evidence for a complex regulating the in vivo activities of early enzymes induced by bacteriophage T4. J Biol Chem. 1974 Dec 10;249(23):7613–7622. [PubMed] [Google Scholar]
- Vanderslice R. W., Yegian C. D. The identification of late bacteriophage T4 proteins on sodium dodecyl sulfate polyacrylamide gels. Virology. 1974 Jul;60(1):265–275. doi: 10.1016/0042-6822(74)90384-5. [DOI] [PubMed] [Google Scholar]
- Velten J., Fukada K., Abelson J. In vitro construction of bacteriophage lambda and plasmid DNA molecules containing DNA fragments from bacteriophage T4. Gene. 1976;1(1):93–106. doi: 10.1016/0378-1119(76)90009-3. [DOI] [PubMed] [Google Scholar]
- Wilson G. G., Tanyashin V. I., Murray N. E. Molecular cloning of fragments of bacteriophage T4 DNA. Mol Gen Genet. 1977 Nov 14;156(2):203–214. doi: 10.1007/BF00283493. [DOI] [PubMed] [Google Scholar]
- Wu R., Geiduschek E. P. Distinctive protein requirements of replication-dependent and -uncoupled bacteriophage T4 late gene expression. J Virol. 1977 Nov;24(2):436–443. doi: 10.1128/jvi.24.2.436-443.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Geiduschek E. P. The role of replication proteins in the regulation of bacteriophage T4 transcription. I. Gene 45 and hydroxymethyl-C-containing DNA. J Mol Biol. 1975 Aug 25;96(4):513–538. doi: 10.1016/0022-2836(75)90137-0. [DOI] [PubMed] [Google Scholar]
- Young E. T., Mattson T., Selzer G., Van Houwe G., Bolle A., Epstein R. Bacteriophage T4 gene transcription studied by hybridization to cloned restriction fragments. J Mol Biol. 1980 Apr 15;138(3):423–445. doi: 10.1016/s0022-2836(80)80011-8. [DOI] [PubMed] [Google Scholar]