Abstract
Selective incorporation of cargo proteins into the forming vesicle is an important aspect of protein targeting via vesicular trafficking. Based on the current paradigm of cargo selection in vesicular transport, proteins to be sorted to other organelles are condensed at the vesicle budding site in the donor organelle, a process that is mediated by the interaction between cargo and coat proteins, which constitute part of the vesicle forming machinery. The cytoplasm to vacuole targeting (Cvt) pathway is an unconventional vesicular trafficking pathway in yeast, which is topologically and mechanistically related to autophagy. Aminopeptidase I (Ape1) is the major cargo protein of the Cvt pathway. Unlike the situation in conventional vesicular transport, precursor Ape1, along with its receptor Atg19/Cvt19, is packed into a huge complex, termed a Cvt complex, independent of the vesicle formation machinery. The Cvt complex is subsequently incorporated into the forming Cvt vesicle. The deletion of APE1 or ATG19 compromised the organization of the pre-autophagosomal structure (PAS), a site that is thought to play a critical role in Cvt vesicle/autophagosome formation. The proper organization of the PAS also required Atg11/Cvt9, a protein that localizes the cargo complex at the PAS. Accordingly, the deletion of APE1, ATG19, or ATG11 affected the formation of Cvt vesicles. These observations suggest a unique concept; in the case of the Cvt pathway, the cargo proteins facilitate receptor recruitment and vesicle formation rather than the situation with most vesicular transport, in which the forming vesicle concentrates the cargo proteins.
The localization of many intracellular proteins is dependent upon movement within transient transport vesicles. The precise transport of proteins via vesicular trafficking is guaranteed by the selective incorporation of cargo into the forming vesicles and specific membrane fusion with an acceptor organelle. The cytoplasm to vacuole targeting (Cvt)1 pathway is an autophagy-related protein targeting pathway in yeast, whereby the resident vacuolar hydrolases, aminopeptidase I (Ape1) and α-mannosidase (Ams1), are directly targeted from the cytoplasm to the vacuole. In this pathway, these two hydrolases are selectively enwrapped by double membrane-bound vesicles, termed Cvt vesicles, followed by fusion with the vacuolar membrane and a breakdown of the inner membrane structure to release precursor Ape1 (prApe1) and Ams1 into the vacuolar lumen (1, 2). The process of the Cvt pathway and autophagy are topologically and mechanistically similar, even though they are different in their physiological functions (3-5). Autophagy is a cellular process responsible for the non-selective bulk degradation of cytoplasmic components in eukaryotic cells, which is induced in response to environmental cues such as nutrient starvation and hormonal stimuli (6, 7). In yeast, nutrient starvation induces the formation of autophagosomes, which are much larger (300-900 nm in diameter) than Cvt vesicles (150 nm in diameter), for efficient transport of cytoplasm to the vacuole. On the other hand, the Cvt pathway is a constitutive biosynthetic pathway highly specific for prApe1 and Ams1. The cargo specificity in the Cvt pathway is conferred by Atg19/Cvt19 that has been characterized as a cargo receptor (8).
Recently, we elucidated the mechanism of cargo selection in the Cvt pathway (9). Two independent processes contributed to the selective incorporation of prApe1 into the Cvt vesicle: the self-assembly of the prApe1 complex, and its recruitment to the perivacuolar vesicle-forming site called the pre-autophagosomal structure (PAS). After synthesis of the prApe1 polypeptide, it rapidly forms dodecamers in the cytosol (10), which further assemble into a higher order structure termed the Ape1 complex; assembly of the Ape1 complex is dependent on the prApe1 propeptide. The Ape1 complex recruits Atg19 through the interaction between the propeptide of prApe1 and a coiled-coil motif of Atg19. Atg11/Cvt9 then binds to the C terminus of Atg19 to target the cargo-receptor complex to the PAS, where the interactions between Atg19 and PAS components ensure the incorporation of the cargo complex into the Cvt vesicle (9). Unlike most receptors that cycle between donor and acceptor membranes, Atg19 is targeted to the vacuole together with cargo proteins and degraded there. The simultaneous binding of another cargo molecule, Ams1, to Atg19 results in an accumulation of Ams1 on the Ape1 complex, allowing an efficient transport of Ams1 to the vacuole. Interestingly, the lack of an Ape1 complex resulted in a dramatic decrease in the turnover of Atg19 as well as a decrease of Ams1 transport to the vacuole (8, 9), suggesting that the Ape1 complex facilitates the incorporation of an Atg19-Ams1 complex into the Cvt vesicles. Because the turnover of Atg19 is dependent on Cvt vesicle formation (8), these results raised a question as to whether the Cvt cargo could also induce Cvt vesicle formation or whether vesicle formation was constitutive and empty vesicles could be formed even without cargo proteins. The role of cargo proteins in facilitating or directing vesicle formation has been difficult to study because in most vesicular trafficking events the full range of cargo molecules have not been defined; in the Cvt pathway, prApe1 appears to be the predominant cargo protein, and even Ams1 is a relatively minor constituent. Even though the Ape1 complex is selectively incorporated into autophagosomes under starvation conditions, neither Ape1 nor Atg19 is required for autophagy (8, 11), suggesting that autophagosome formation does not depend on the presence of specific cargo components. In this study, we show that the cargo-receptor complex facilitates the formation of Cvt vesicles but not autophagosomes.
EXPERIMENTAL PROCEDURES
Strains, Plasmids, and Media
The Saccharomyces cerevisiae yeast strains used in this study are listed in Table I. A plasmid expressing GFP-Atg8 was based on pRS306 containing the GFP-ATG8 gene with the endogenous ATG8 promoter (12) and used for yeast transformation to integrate GFP-ATG8 at the URA3 locus. The pRS316 GFP-APG1 plasmid and YCplac33 GFP-APG2 (pTS112) (13) were kind gifts from Dr. Yoshinori Ohsumi (National Institute for Basic Biology, Okazaki, Japan). The atg1ts allele (12) was cloned on pRS414. Yeast cells were grown in SCD medium (0.67% yeast nitrogen base without amino acids, 0.5% casamino acid, and 2% glucose) supplemented with 0.003% adenine, 0.005% tryptophan, and 0.002% uracil, if necessary. For nitrogen starvation, SD(-N) medium (0.17% yeast nitrogen base without ammonium sulfate and amino acids and 2% glucose) was used.
TABLE I.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| SEY6210 | MATα his3-Δ200 leu2-3,112 lys2-801 trp1-Δ901 ura3-52 suc2-Δ9 GAL | Ref. 34 |
| FRY138 | SEY6210; ATG9-YFP::HIS5 S.p. atg1Δ::URA3 | Ref. 27 |
| TYY014 | SEY6210; ATG20-YFP::HIS5 S.p. vps38Δ::LEU2 K.l. atg11Δ::URA3 K.l. | This study |
| YTS135 | SEY6210; ATG9-YFP::HIS5 S.p. atg1Δ::URA3 atg19Δ::LEU2 K.l. | This study |
| YTS136 | SEY6210; ATG9-YFP::HIS5 S.p. atg1Δ::URA3 ape1 ::LEU2 | This study |
| YTS150 | SEY6210; ATG9-YFP::HIS5 S.p. atg1Δ::URA3 atg11Δ::LEU2 K.l. | This study |
| YTS180 | SEY6210; ATG20-YFP::HIS5 S.p. vps38Δ::LEU2 K.l. | This study |
| YTS184 | SEY6210; ATG20-YFP::HIS5 S.p. vps38Δ::LEU2 K.l. atg19Δ::URA3 K.l. | This study |
| YTS185 | SEY6210; ATG20-YFP::HIS5 S.p. vps38Δ::LEU2 K.l. ape1Δ::TRP1 | This study |
| YTS187 | SEY6210; URA3::GTP-ATG8 | This study |
| YTS188 | SEY6210; atg1Δ::HIS5 S.p. GFP-ATG8:: URA3 | This study |
| YTS189 | SEY6210; pep4Δ:: LEU2 GFP-ATG8::URA3 | This study |
| YTS190 | SEY6210; atg19Δ::HIS5 S.p. GFP-ATG8::URA3 | This study |
| YTS191 | SEY6210; ape1Δ::LEU2 GFP-ATG8::URA3 | This study |
| YTS192 | SEY6210; atg11Δ::HIS3 GFP-ATG8::URA3 | This study |
| YTS193 | SEY6210; atg2Δ::HIS5 S.p. [GFP-ATG2 URA3] | This study |
| YTS194 | SEY6210; atg2Δ::HIS5 S.p. atg19 ::LEU2 K.l. [GFP-ATG2 URA3] | This study |
| YTS195 | SEY6210; atg2Δ::HIS5 S.p. ape1 ::LEU2 [GFP-ATG2 URA3] | This study |
| YTS196 | SEY6210; atg2Δ::HIS5 S.p. atg11 ::LEU2 K.l. GFP-ATG2 URA3 | This study |
| YTS197 | SEY6210 [GFP-ATG1 URA3] | This study |
| YTS198 | SEY6210; atg19Δ::HIS5 S.p. [GFP-ATG1 URA3] | This study |
| YTS199 | SEY6210; ape1Δ::LEU2 [GFP-ATG1 URA3] | This study |
| YTS200 | SEY6210; atg11Δ::HIS3 [GFP-ATG1 URA3] | This study |
| YTS201 | SEY6210; atg1Δ::HIS5 S.p. URA3::GFP-ATG8 [atg1tsTRP1] | This study |
| YTS202 | SEY6210; atg1Δ::HIS5 S.p. atg19 ::LEU2 K.l. URA3::GFP-ATG8 [atg1tsTRP1] | This study |
| YTS203 | SEY6210; atg1Δ::HIS5 S.p. ape1 ::LEU2 URA3::GFP-ATG8 [atg1tsTRP1] | This study |
| YTS204 | SEY6210; atg1Δ::HIS5 S.p. atg11Δ::LEU2 K.l. URA3::GFP-ATG8 [atg1tsTRP1] | This study |
Transport of GFP-Atg8 to the Vacuole
The strains harboring the atg1ts allele were grown in SCD medium at 37 °C overnight to A600 = 1.0. The cultures were divided into two tubes, and either rapamycin (0.2 μg/ml) or the drug vehicle was added to each tube. After incubation at 37 °C for 10 min, the tubes were placed at 30 °C to activate the Cvt pathway or autophagy. At various time points, 1 ml of culture was harvested and used to prepare a protein extract. Protein extracts equivalent to A600 = 0.1 unit of yeast cells were subjected to SDS-PAGE and probed with anti-Ape1 antiserum and anti-GFP antibody (Covance Research Products, Berkeley, CA).
Fluorescence Microscopy
Yeast cells expressing fluorescent protein-fused chimeras were grown in SCD medium to mid-log phase. For nitrogen starvation, the cells grown in SCD medium were washed with water twice and resuspended in SD(-N) medium. The cells were observed with a fluorescence microscope as described previously (14).
RESULTS
Atg8 Can Be Used to Trace Cvt Vesicle Formation
Atg19 is a receptor protein for the vacuolar hydrolases Ape1 and Ams1 in the Cvt pathway. It is transported to the vacuole together with cargo proteins and degraded within the vacuole lumen. Previously, we found that the turnover of Atg19 and the transport of Ams1 were severely affected by a lack of Ape1 (9). Because both Atg19 turnover and Ams1 transport are also dependent on the proper function of the Cvt pathway, we asked whether the Ape1 complex could induce the formation of Cvt vesicles or whether empty vesicles could be formed without the Ape1 complex. To analyze the effect of a lack of cargo complex on the formation of Cvt vesicles, we needed to develop a suitable marker protein. Precursor Ape1 is the standard marker used to monitor delivery via the Cvt and autophagy pathways; however, we were unable to use Ape1 as a marker because our goal was to examine vesicle formation in the absence of this specific cargo protein. Among the characterized Atg proteins, only Atg19 and Atg8/Aut7 remain associated with the completed Cvt vesicle/autophagosome. Atg19 was not suitable as a marker protein because it functions in cargo recognition. Accordingly, we used Atg8 as a marker of the Cvt pathway. Atg8 is essential for Cvt vesicle formation and for expansion of the autophagosome membrane (15-17). Similar to Atg19, Atg8 is delivered to the vacuole as a component of the Cvt vesicle/autophagosome and is degraded after breakdown of the inner vesicle within the vacuole lumen. Accordingly, Cvt vesicle/autophagosome formation can be assessed by measuring the amount of Atg8 protein delivered to the vacuole. Using Atg8 to monitor vesicle formation, however, presented a significant problem for analyzing the Cvt pathway; Atg8 is synthesized at very low levels under vegetative conditions and is only induced upon starvation. Accordingly, it is problematic to monitor delivery of Atg8 under vegetative conditions through a radioactive pulse/chase analysis; the level of protein delivered to the vacuole is too low to allow an accurate quantification, and it is inherently difficult to follow the kinetics of vacuolar delivery by examining loss of a protein.
Because of these technical difficulties, we designed an alternate strategy to monitor vesicle formation. GFP tagging of Atg8 has been used to trace the process of the Cvt pathway and autophagy (12, 18). Even though Atg8 is subject to degradation upon vacuolar delivery, the relative stability of GFP to the activity of vacuolar hydrolases led us to propose that the GFP moiety would accumulate in the vacuolar lumen as a consequence of the transport of GFP-Atg8 to the vacuole; the accumulated GFP could be easily monitored and would reflect delivery of Atg8. To confirm the suitability of this approach, we performed immunoblot analysis of GFP-Atg8 using anti-GFP antibody. In wild type cells expressing GFP-Atg8 grown in rich medium, we detected protein bands of 40 and 26 kDa (Fig. 1A, bottom panel). These molecular masses correspond to the predicted sizes of full-length GFP-Atg8 and free GFP. As controls, we examined atg1Δ/agp1Δ and pep4Δ strains, which are defective in Cvt vesicle/autophagosome formation and the breakdown of intravacuolar vesicles, respectively. In contrast to the result seen in wild type cells, only full-length GFP-Atg8 was seen in the mutant strains (Fig. 1A, bottom panel). These results indicated that the generation of free GFP was dependent on the proper function of the Cvt pathway. When autophagy was induced by incubating the cells in starvation medium, the transport of GFP-Atg8 was enhanced in wild type cells, whereas again no liberation of GFP was observed in atg1Δ or pep4Δ mutant cells (Fig. 1A, bottom panel). These results support the idea that an increased level of Atg8 is required to form autophagosomes (17). An analysis of Ape1 from the same protein extracts verified that the mutant strains displayed the expected phenotypes with regard to prApe1 processing (Fig. 1A, top panel).
FIG.1.
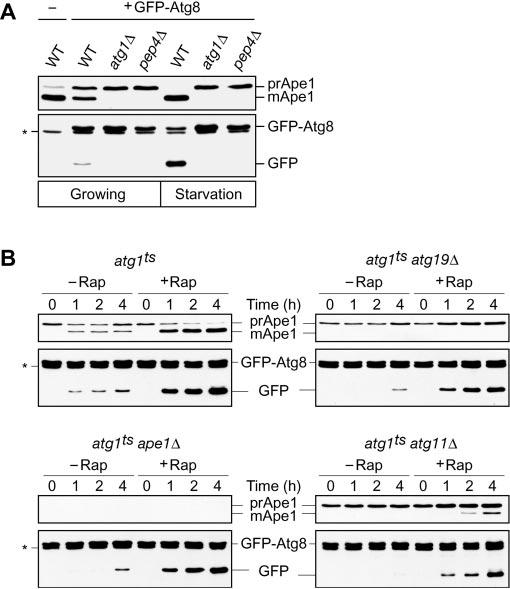
The Cvt cargo-receptor complex is required for efficient delivery of Atg8 to the vacuole. A, the generation of free GFP from GFP-Atg8 reflects delivery through the Cvt and autophagy pathways. The wild type, atg1Δ, and pep4Δ cells expressing GFP-Atg8 (YTS187, YTS188, and YTS189, respectively) were grown in SCD (rich medium; growing conditions) to A600 = 1.0 and starved in SD(-N) for 3 h. Cell lysates equivalent to A600 = 0.1 unit of cells were subjected to immunoblot analysis with anti-Ape1 antiserum and anti-GFP antibody. B, delivery of Atg8 to the vacuole was blocked or extensively delayed in the absence of Ape1, Atg19, and Atg11. The atg1ts, atg1ts ape1Δ, atg1ts atg19Δ, and atg1ts atg11Δ cells expressing GFP-Atg8 (YTS201, YTS203, YTS202, and YTS204, respectively) were grown in SCD to A600 = 1.0 at a non-permissive temperature of 37 °C. After treatment with or without 0.2 μg/ml rapamycin (Rap) for 10 min at 37 °C, the cells were transferred to a permissive temperature of 30 °C and incubated for the indicated times. Ape1 and GFP-Atg8 were analyzed by immunoblotting as indicated in A. The positions of precursor and mature Ape1 (mApe1) and of full-length GFP-Atg8 and free GFP are indicated. The asterisk marks a nonspecific cross-reacting band.
To allow a kinetic analysis of GFP-Atg8 transport, we took advantage of a conditional atg1 mutant. Cells harboring a temperature-sensitive allele of ATG1, atg1ts, and expressing GFP-Atg8 were grown to mid-log phase at non-permissive temperature and then shifted to permissive temperature to activate the Cvt pathway, followed by immunoblot analysis with anti-Ape1 antiserum and anti-GFP antibody. After shifting to the permissive temperature, prApe1 was converted to the mature form (mApe1) within 1 h, indicating that prApe1 reached the vacuole immediately after the atg1ts block was released (Fig. 1B, top left, top panel). Similarly, the conversion of GFP-Atg8 to GFP occurred within 1 h after incubation at 30 °C, and accumulation of GFP gradually increased during the time course (Fig. 1B, top left, bottom panel). The addition of rapamycin to the culture, which mimics nutrient starvation conditions, resulted in an enhanced transport of GFP-Atg8 to the vacuole (Fig. 1B, top left, bottom panel). These results confirmed that this system is useful for a kinetic analysis of Cvt vesicle/autophagosome formation.
Absence of the Cvt Cargo-Receptor Complex Impairs Cvt Vesicle but Not Autophagosome Formation
To investigate whether the Cvt cargo complex is involved in Cvt vesicle formation, we carried out kinetic analyses using an atg1ts ape1Δ double mutant strain expressing GFP-Atg8. After growth at 37 °C, the cells were shifted to 30 °C to activate the Cvt pathway. In the absence of rapamycin treatment, the generation of free GFP was severely delayed up to 4 h after incubation at 30 °C, suggesting that prApe1 is required for proper or efficient Cvt vesicle formation (Fig. 1B, bottom left, bottom panel). The receptor protein Atg19 is concentrated on the Ape1 complex and behaves like an adaptor protein to connect the Ape1 complex and the vesicle forming machinery; the Atg19-Ape1 complex is termed the Cvt complex. Thus, we hypothesized that Atg19 might be also important for Cvt vesicle formation. To test this, we carried out the same analysis with an atg1ts atg19Δ strain. As expected, GFP-Atg8 transport was delayed in this strain and showed kinetics similar to that seen in the atg1ts ape1Δ strain (Fig. 1B, top right, bottom panel). These results suggested that the cargo-receptor complex induces the formation of the Cvt vesicle rather than being incorporated within a constitutively forming vesicle.
In contrast to the block in Cvt vesicle formation seen in rich medium, the deletion of either APE1 or ATG19 did not affect autophagosome formation when cells were treated with rapamycin to induce autophagy (Fig. 1B, bottom left and top right, bottom panels). This result was consistent with previous observations obtained by using autophagy-dependent activation of alkaline phosphatase activity (8, 11). It is believed that Cvt vesicles and autophagosomes are formed at the PAS, where most of the Atg proteins localize (11, 12, 19). The Cvt complex also localizes to this structure (9, 11). To examine whether proper localization of the cargo-receptor complex to the PAS is also important for vesicle formation, we constructed an atg1ts atg11Δ/cvt9Δ strain expressing GFP-Atg8. Atg11/Cvt9 is a specific factor for the Cvt pathway and pexophagy (20) and is required to localize the cargo-receptor complex to the PAS in the Cvt pathway (9). In this strain, no transport of GFP-Atg8 was observed without rapamycin treatment, whereas with rapamycin the transport of GFP-Atg8 was comparable with that of other strains including the wild type (Fig. 1B). This result suggested that cargo recruitment to the PAS was also important for Cvt vesicle formation but not for autophagosome formation. Examination of Ape1 again confirmed the expected phenotype of the various strains. In particular, prApe1 was absent in the ape1Δ strain, showed normal maturation in the atg1ts strain at the permissive temperature, accumulated in the absence of Atg19, and was partially matured in the atg11Δ strain only under starvation conditions (Fig. 1B, top panels).
The Cvt Complex Is Important for PAS Organization under Growing Conditions
The PAS is thought to be an organizing center for Cvt vesicle/autophagosome formation based on several lines of evidence: (i) most of the Atg proteins are located at the PAS under both growing and starvation conditions (12, 14, 19); (ii) the localization of some Atg proteins at the PAS is dependent on the function of other Atg proteins (12, 13, 18, 21); and (iii) GFP-Atg8 transiently localizes at the PAS and is then transported to the vacuole (12, 14). Accordingly, we decided to examine the effect of depletion of the cargo complex on the organization of the PAS. First, we examined the localization of GFP-Atg8 under growing conditions. In a wild type strain, 25% of the cells contained a single GFP-Atg8 punctate signal or a few GFP-Atg8 punctate signals at the PAS (Fig. 2). In contrast, the PAS localization of GFP-Atg8 was observed in only 6.0% and 2.5% of ape1Δ and atg19Δ cells, respectively (Fig. 2). The atg11Δ strain also showed a severe defect in GFP-Atg8 localization at the PAS; only 3.3% of the cells had punctate signals of GFP-Atg8 (Fig. 2). These results suggested that the cargoreceptor complex and its recruitment to the PAS were required for the proper localization of Atg8 at the PAS under growing conditions. On the other hand, these mutant strains did not show any difference in GFP-Atg8 localization compared with the wild type strain under starvation conditions; most of the cells displayed GFP staining in their vacuoles, and about 75-90% of the cells showed a punctate dot of GFP-Atg8 at the PAS (Fig. 2). This result was consistent with previous observations that Ape1, Atg19, and Atg11 are dispensable for autophagy (8, 11, 20) (Fig. 1) and suggested that the PAS localization of Atg8 occurred independently of the recognition of the Cvt cargo under starvation conditions. It was reported that the PAS localization of Atg8 is dependent on its lipidation state (12). Therefore, we examined the lipidation of Atg8 in ape1Δ, atg19Δ, and atg11Δ cells and found there was no difference in lipidation between the wild type and these mutant strains (data not shown), suggesting that Atg8 still localized on some membrane structure, although it was not concentrated at the PAS in these mutant cells.
FIG.2.
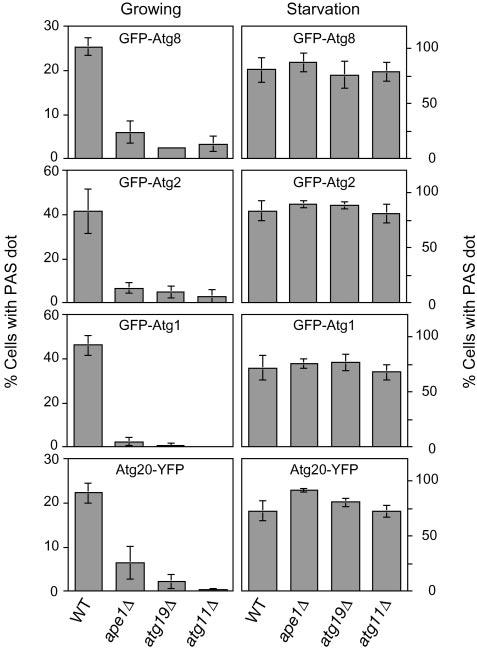
Quantification of cells containing pre-autophagosomal structures. The wild type, ape1Δ, atg11Δ, and atg19Δ cells expressing GFP-Atg8 (YTS187, YTS190, YTS191, and YTS192), GFP-Atg2 (YTS193, YTS194, YTS195, and YTS196), GFP-Atg1 (YTS197, YTS198, YTS199, and YTS200), or Atg20-YFP (YTS180, YTS184, YTS185, and TYY014) were grown in SCD (rich medium; growing conditions) to A600 = 1.0 and starved in SD(-N) for 1 h. For each strain, 150-250 cells containing fluorescent punctate dots were quantified by fluorescence microscopy. Error bars indicate the S.D. of three independent experiments. The chromosomal VPS38 gene was deleted in the cells expressing Atg20-YFP to eliminate punctate localization at the endosome (see text for details). Punctate localization of GFP-Atg8, GFP-Atg2, GFP-Atg1, and Atg20-YFP to the PAS was greatly diminished in the absence of the Cvt cargo-receptor complex under growing conditions, but not under starvation conditions.
Because Atg8 physically interacts with Atg19 (9), we wondered whether only Atg8 lost its localization at the PAS or whether the overall organization of the PAS was compromised in the absence of the Cvt cargo. Recently, it was reported that the PAS localization of Atg2/Apg2 is important for its function in Cvt vesicle/autophagosome formation (13, 22). Because its localization at the PAS is independent of Atg8, we selected Atg2 as a second PAS marker to examine the organization of the PAS. In rich medium, about 40% of wild type cells showed the PAS localization of GFP-Atg2, whereas it was decreased to <7% in ape1Δ, atg19Δ, and atg11Δ strains (Fig. 2). In contrast, the PAS localization of GFP-Atg2 in these strains was comparable with that in the wild type strain in a starvation medium (Fig. 2). A similar result was obtained with a GFP-Atg1 construct (Fig. 2); Atg1 is a serine/threonine kinase that is required for both the Cvt pathway and autophagy (23). These results indicated that the cargo-dependent localization of Atg2 or Atg1 at the PAS was seen only under growing conditions; the deletion of APE1, ATG19, or ATG11 did not result in any defect in the localization of GFP-Atg2 or GFP-Atg1 under starvation conditions.
One of the key components that is thought to play an important role in the initial stage of PAS formation is the phosphatidylinositol (PI) 3-kinase complex that includes Vps34, Vps15, and Vps30/Atg6 (24). Function of the PI 3-kinase complex I, which includes Atg14/Apg14 and acts at the PAS, is required for recruitment of the Cvt pathway-specific factors Atg20/Cvt20 and Atg24/Cvt13. These proteins localize at the PAS dependent on their phox homology domains that bind to PI 3-phosphate (25). The Atg20 and Atg24 proteins are also implicated in protein retrieval from the early endosome to the late Golgi and primarily localize at the endosome for this function (26). The PAS localization of Atg20 and Atg24 is affected by deleting ATG14 encoding a Cvt/autophagy pathway-specific subunit of PI 3-kinase complex I, whereas their endosomal localization is lost by deletion of VPS38 encoding a carboxypeptidase Y pathway-specific subunit of PI 3-kinase complex II (25, 26). We decided to extend our analysis by examining the role of the Cvt cargo and cargo packaging machinery in the localization of factors dependent on PI 3-kinase function. Accordingly, we decided to use a vps38Δ strain to analyze the Cvt pathway specific localization of Atg20. First, we confirmed that the punctate dots of Atg20-YFP observed in vps38Δ cells colocalized with CFP-Atg8 and CFP-Ape1 as markers of the PAS (data not shown). About 22% of the vps38Δ cells contained dot structures of Atg20-YFP corresponding to the PAS under growing conditions (Fig. 2). Similar to other PAS proteins, the PAS localization of Atg20-YFP was dramatically decreased in vps38Δ ape1Δ, vps38Δ atg19Δ, and vps38Δ atg11Δ strains under growing conditions (Fig. 2). Interestingly, Atg20-YFP still localized at the PAS under starvation conditions, even though Atg20 is not required for autophagy; more than 70% of the vps38Δ cells possessed the PAS dots of Atg20-YFP after incubation in a starvation medium (Fig. 2). The additional deletion of APE1, ATG19, or ATG11 had essentially no affect on the PAS localization of Atg20-YFP under autophagy conditions (Fig. 2). Taken together with the observations for other Atg proteins, these results suggested that the cargo-receptor complex might be important for the overall organization of the PAS rather than for the localization of an individual Atg protein at the PAS under growing conditions.
Recently, it has been reported that the Atg1-Atg13/Apg13 protein kinase complex is essential for cycling of the transmembrane protein Atg9/Apg9 between the PAS and other cytosolic punctate structures (27). Atg9 localizes to the PAS and the other cytosolic punctate structures in wild type cells (12), whereas its localization is restricted at the PAS in atg1Δ or atg13Δ cells (27), suggesting that the Atg1-Atg13 protein kinase complex regulates the retrieval transport of Atg9 from the PAS. Accordingly, we examined whether this restriction of Atg9 localization at the PAS in atg1Δ cells was perturbed by a lack of the Cvt complex. In atg1Δ cells, Atg9-YFP was restricted at the PAS as observed previously (27) (Fig. 3). The additional deletion of APE1 or ATG19 resulted in a scattered localization of Atg9-YFP (Fig. 3), suggesting that the cargoreceptor complex is required for the sorting of Atg9 to the PAS under nutrient-rich conditions. After incubating atg1Δ ape1Δ or atg1Δ atg19Δ cells in starvation medium for 1 h, Atg9-YFP accumulated as a prominent dot, although several tiny dots were still observed (Fig. 3).
FIG.3.
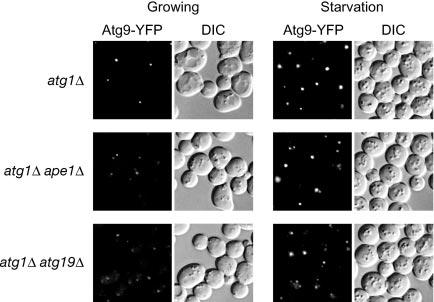
Atg9 cycling requires the Cvt cargo-receptor complex under growing conditions. The atg1Δ, atg1Δ ape1Δ, and atg1Δ atg19Δ cells expressing Atg9-YFP (FRY138, YTS136, and YTS135, respectively) were grown in SCD (rich medium; growing conditions) and then shifted to SD(-N) (starvation conditions) and observed by fluorescence microscopy. Atg9 normally transits to the PAS and is retrieved from this site in an Atg1-dependent manner. Delivery to the PAS was defective in the absence of the Cvt cargo-receptor complex.
DISCUSSION
Selective incorporation of cargo proteins into the forming vesicle is an important aspect of protein targeting via vesicular trafficking. According to the paradigm of cargo selection in vesicular transport, the association of cargo proteins with coat proteins (a key component in the vesicle forming machinery) leads to their concentration at the vesicle budding site in the donor organelle (28). We have investigated the mechanism of cargo selection in the Cvt pathway that is topologically and mechanistically similar to autophagy (8, 9). Unlike the situation in conventional vesicular transport, the Cvt cargo proteins are concentrated as a huge complex, the Cvt complex, which is composed of the resident vacuolar hydrolases Ape1 and Ams1 and the receptor protein Atg19, in a process independent of the vesicle formation machinery, before they are incorporated into a double membrane Cvt vesicle (9). In this study, we propose an unusual concept for cargo selection in vesicular transport: the cargo proteins facilitate the process of vesicle formation rather than concentration of the cargo and incorporation into the forming vesicle through the action of the vesicle forming machinery.
In wild type cells, at least some population of most of the Atg proteins is present at the pre-autophagosomal structure. In contrast, in the absence of Ape1, Atg19, and Atg11, there is nearly a complete block in PAS formation based on fluorescence microscopy of tagged proteins. The best-characterized marker protein that appears to be a structural component of the PAS and the resulting sequestering vesicle is Atg8 conjugated to phosphatidylethanolamine (PE). In most strains bearing mutations in components of the vesicle forming machinery, Atg8-PE is typically localized to the PAS; in wild type cells, Atg8-PE is additionally delivered to the vacuole and degraded. Atg8 is induced by starvation but is present at relatively low levels under vegetative conditions. Accordingly, it is difficult to quantify Atg8-PE delivery to the vacuole via the Cvt pathway. We developed a method to monitor delivery of Atg8-PE to the vacuole to allow quantification of Cvt vesicle formation. Our approach relied on the stability of GFP and the instability of Atg8 within the vacuole lumen. We used a GFP-Atg8 fusion to monitor release of free GFP after vacuolar delivery. The appearance of free GFP was dependent on vacuolar hydrolase activity and Atg/Cvt proteins, indicating that it reflected the normal Cvt and autophagy pathways (Fig. 1).
The assembly of the Cvt complex and its recruitment to the priming site of Cvt vesicle/autophagosome formation led to the convergence of Atg proteins at this site to organize the PAS under growing conditions (Figs. 2 and 3). Therefore, we concluded that the pre-concentrated cargo complex might attract the vesicle forming machinery to it causing it to be surrounded by the Cvt vesicle. This model provides a reasonable explanation as to why the Cvt vesicle completely excludes cytoplasmic material (4); that is, the Cvt vesicle does not randomly engulf cytosol but rather nucleates around the cargo complex. It seems that a membrane structure is also enriched on the cargo complex together with Atg proteins because the sorting of the transmembrane protein Atg9 (29) to the PAS was also dependent on the cargo complex (Fig. 3). Atg8-PE also behaves like an integral membrane protein (16, 30), and its PAS localization is dependent on its lipidation (12, 18). Although the state of Atg8 lipidation in ape1Δ, atg19Δ, or atg11Δ strains was comparable to that in the wild type strain (data not shown), there was less PAS localization in these mutants, suggesting that Atg8-PE was localized on some membrane structure other than the PAS. This unidentified membranous structure could contain other Atg proteins and become enriched on the cargo complex in a process mediated by Atg11; Atg11 connects the Cvt complex and the Atg machinery (9, 23). With regard to the source of membrane for the Cvt vesicle and autophagosome, there have been arguments for the requirement of the endoplasmic reticulum (1, 31, 32). Recently, it has been reported that the early stage of the secretory pathway (endoplasmic reticulum and/or Golgi complex) is required for both Cvt vesicle and autophagosome formation (33). Overall, these studies suggest that the Atg/Cvt protein-localizing membrane structure might be supplied from the early secretory pathway. In contrast to the case under growing conditions, the assembly of the Cvt complex is not required for the formation of the autophagosome (8, 11) (Fig. 1) or the proper organization of the PAS under conditions of nutrient starvation (Fig. 2). These results suggest that autophagy uses a mechanism different from the Cvt pathway to organize the PAS, and the details of this mechanism remain to be solved.
The deletion of ATG11 resulted in a more severe defect in Cvt vesicle formation than that seen with deletions of APE1 or ATG19 (Fig. 1), presumably because Atg11 functions in another transport pathway other than the Cvt pathway. It is known that excess peroxisomes are selectively degraded by autophagy, or pexophagy, in an Atg11-dependent manner (20). According to its function in the Cvt pathway, Atg11 might act as an adaptor protein for peroxisomes that is required for their selective degradation. Even under normal growth conditions, a basal level of peroxisome degradation could occur to maintain normal levels of peroxisomes or to remove damaged peroxisomes, which might lead to the formation of vesicles containing Atg8 to sequester this organelle. Therefore, the delivery of GFP-Atg8 was completely blocked in atg11Δ cells in which both the Cvt pathway and pexophagy are impaired (Fig. 1).
Autophagy is an inducible pathway, whereas the Cvt pathway is considered to be constitutive, but what does this mean with regard to vesicle formation? In our study, we have focused on the connection between the cargo and the formation of the sequestering vesicle in the Cvt pathway. The Cvt pathway represents a unique type of vesicle-mediated trafficking process in that the cargo forms a complex independent of its receptor, and the cargo-receptor complex induces the formation of the sequestering vesicle. There must be an additional level of control, however, during the vesicle formation process. When precursor Ape1 is overexpressed, the Cvt complex is enlarged, and a portion of the protein accumulates in the cytosol (1, 4); the vesicle forming machinery is apparently unable to completely enwrap the enlarged complex, suggesting that the membrane does not simply wrap around the Cvt complex that acts as a scaffold. It is possible that the limited supply of membrane and/or Atg proteins that is available to the Cvt pathway under vegetative conditions is not sufficient to enwrap the larger Cvt complex. Cvt vesicles are of a carefully defined size relative to autophagosomes. Whereas transport vesicles that bud off from pre-existing organelles are also of strictly defined sizes, this is presumably due to constraints imposed on vesicle curvature by coat components. The action of a transient coat has not yet been established for the Cvt and autophagy pathways. Further work will be needed to determine additional details about the process of sequestering vesicle formation in these pathways.
Footnotes
This work was supported by United States Public Health Service Grant GM53396 from the National Institutes of Health (to D. J. K.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
- Cvt
- cytoplasm to vacuole targeting
- PAS
- pre-autophagosomal structure
- GFP
- green fluorescent protein
- PI
- phosphatidylinositol
- YFP
- yellow fluorescent protein
- PE
- phosphatidylethanolamine
- Ape1
- aminopeptidase I
- Ams1
- α-mannosidase
- prApe1
- precursor Ape1.
REFERENCES
- 1.Klionsky DJ, Cueva R, Yaver DS. J. Cell Biol. 1992;119:287–299. doi: 10.1083/jcb.119.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutchins MU, Klionsky DJ. J. Biol. Chem. 2001;276:20491–20498. doi: 10.1074/jbc.M101150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. J. Cell Biol. 1992;119:301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba M, Osumi M, Scott SV, Klionsky DJ, Ohsumi Y. J. Cell Biol. 1997;139:1687–1695. doi: 10.1083/jcb.139.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott SV, Baba M, Ohsumi Y, Klionsky DJ. J. Cell Biol. 1997;138:37–44. doi: 10.1083/jcb.138.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klionsky DJ, Ohsumi Y. Annu. Rev. Cell Dev. Biol. 1999;15:1–32. doi: 10.1146/annurev.cellbio.15.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Klionsky DJ, Emr SD. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott SV, Guan J, Hutchins MU, Kim J, Klionsky DJ. Mol. Cell. 2001;7:1131–1141. doi: 10.1016/s1097-2765(01)00263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shintani T, Huang W-P, Stromhaug PE, Klionsky DJ. Dev. Cell. 2002;3:825–837. doi: 10.1016/s1534-5807(02)00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, Scott SV, Oda MN, Klionsky DJ. J. Cell Biol. 1997;137:609–618. doi: 10.1083/jcb.137.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki K, Kamada Y, Ohsumi Y. Dev. Cell. 2002;3:815–824. doi: 10.1016/s1534-5807(02)00359-3. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. EMBO J. 2001;20:5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shintani T, Suzuki K, Kamada Y, Noda T, Ohsumi Y. J. Biol. Chem. 2001;276:30452–30460. doi: 10.1074/jbc.M102346200. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Huang W-P, Stromhaug PE, Klionsky DJ. J. Biol. Chem. 2002;277:763–773. doi: 10.1074/jbc.M109134200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, Ohsumi Y. J. Cell Biol. 1999;147:435–446. doi: 10.1083/jcb.147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W-P, Scott SV, Kim J, Klionsky DJ. J. Biol. Chem. 2000;275:5845–5851. doi: 10.1074/jbc.275.8.5845. [DOI] [PubMed] [Google Scholar]
- 17.Abeliovich H, Dunn WA, Jr., Kim J, Klionsky DJ. J. Cell Biol. 2000;151:1025–1034. doi: 10.1083/jcb.151.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Huang W-P, Klionsky DJ. J. Cell Biol. 2001;152:51–64. doi: 10.1083/jcb.152.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noda T, Suzuki K, Ohsumi Y. Trends Cell Biol. 2002;12:231–235. doi: 10.1016/s0962-8924(02)02278-x. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Kamada Y, Stromhaug PE, Guan J, Hefner-Gravink A, Baba M, Scott SV, Ohsumi Y, Dunn WA, Jr., Klionsky DJ. J. Cell Biol. 2001;153:381–396. doi: 10.1083/jcb.153.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan J, Stromhaug PE, George MD, Habibzadegah-Tari P, Bevan A, Dunn WA, Jr., Klionsky DJ. Mol. Biol. Cell. 2001;12:3821–3838. doi: 10.1091/mbc.12.12.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C-W, Kim J, Huang W-P, Abeliovich H, Stromhaug PE, Dunn WA, Jr., Klionsky DJ. J. Biol. Chem. 2001;276:30442–30451. doi: 10.1074/jbc.M102342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, Ohsumi Y. J. Cell Biol. 2000;150:1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kihara A, Noda T, Ishihara N, Ohsumi Y. J. Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nice DC, Sato TK, Stromhaug PE, Emr SD, Klionsky DJ. J. Biol. Chem. 2002;277:30198–30207. doi: 10.1074/jbc.M204736200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hettema EH, Lewis MJ, Black MW, Pelham HRB. EMBO J. 2003;22:548–557. doi: 10.1093/emboj/cdg062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reggiori F, Tucker KA, Stromhaug PE, Klionsky DJ. Dev. Cell. 2004;6:79–90. doi: 10.1016/s1534-5807(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 28.Bonifacino JS, Glick BS. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 29.Noda T, Kim J, Huang W-P, Baba M, Tokunaga C, Ohsumi Y, Klionsky DJ. J. Cell Biol. 2000;148:465–480. doi: 10.1083/jcb.148.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, Ohsumi M, Takao T, Noda T, Ohsumi Y. J. Cell Biol. 2000;151:263–276. doi: 10.1083/jcb.151.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishihara N, Hamasaki M, Yokota S, Suzuki K, Kamada Y, Kihara A, Yoshimori T, Noda T, Ohsumi Y. Mol. Biol. Cell. 2001;12:3690–3702. doi: 10.1091/mbc.12.11.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamasaki M, Noda T, Ohsumi Y. Cell Struct. Funct. 2003;28:49–54. doi: 10.1247/csf.28.49. [DOI] [PubMed] [Google Scholar]
- 33.Reggiori F, Wang C-W, Nair U, Shintani T, Abeliovich H, Klionsky DJ. Mol. Biol. Cell. 2004;15:2189–2204. doi: 10.1091/mbc.E03-07-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson JS, Klionsky DJ, Banta LM, Emr SD. Mol. Cell. Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]


