Abstract
The biogenic amines octopamine and tyramine are believed to play a number of important roles in the behavior of invertebrates including the regulation of motor function. To investigate the role of octopamine and tyramine in locomotor behavior in honey bees, subjects were injected with a range of concentrations of octopamine, tyramine, mianserin or yohimbine. Continuous observation of freely moving worker bees was used to examine the effects of these treatments on the amount of time honey bees spent engaged in different locomotor behaviors such as walking, grooming, fanning and flying. All treatments produced significant shifts in behavior. Decreases in time spent walking and increases in grooming or stopped behavior were observed for every drug. However, the pattern of the shift depended on drug, time after injection and concentration. Flying behavior was differentially effected with increases in flying seen in octopamine treated bees, whereas those receiving tyramine showed a decrease in flying. Taken together, these data provide evidence that octopamine and tyramine modulate motor function in the honey bee perhaps via interaction with central pattern generators or through effects on sensory perception.
Keywords: Trace amines, grooming, flying, central pattern generator, biogenic amines
Introduction
The biogenic amines are a class of intercellular signaling molecules that act as neurotransmitters, neuromodulators and neurohormones. Two biogenic amines found at relatively high levels in the invertebrate nervous system are octopamine and tyramine. Tyramine is synthesized from the amino acid tyrosine via the enzyme tyrosine decarboxylase. Tyramine is then, in turn, converted to octopamine by tyramine β-hydroxylase. Until recently, tyramine was considered only to be the precursor of octopamine. However, it is now well established that tyramine is also a neuroactive substance in its own right (Alkema et al., 2005; Kutsukake et al., 2000; Nagaya et al., 2002).
Octopamine and tyramine have been linked to modulation of a diverse range of behaviors. For example, Drosophila larvae with elevated tyramine and reduced octopamine levels exhibit abnormal locomotion (Saraswati et al., 2004) perhaps due to defects in motor pattern generation (Fox et al., 2006). Recent isolation of tyrosine decarboxylase and tyramine β-hydroxylase mutations in C. elegans has provided more evidence that octopamine and tyramine play distinct roles in egg laying and locomotor behaviors (Alkema et al., 2005). Octopamine and tyramine also have roles in non-locomotor behavior, such as the modulation of sensory perception (Erber and Kloppenburg, 1995; Kutsukake et al., 2000; Scheiner et al., 2002) and the role of octopamine in associative learning (Hammer and Menzel, 1998; Schwaerzel et al., 2003). In honey bees, changes in the levels of these molecules correlate with the shift in behavior from in hive tasks, such as taking care of the brood, to outdoor activities, such as foraging (Bozic and Woodring, 1998; Harris and Woodring, 1992; Schulz and Robinson, 1999; Wagener-Hulme et al., 1999).
The actions of octopamine and tyramine are mediated through G protein coupled receptors expressed on the surface of responsive cells. (For recent reviews see: Blenau and Baumann, 2001, 2003.) Receptors specific for either octopamine (AmOA1) or tyramine (AmTYR1) have been cloned and characterized from the honey bee. When AmOA1receptors are expressed in HEK cells, activation of the receptor leads to oscillations in intracellular Ca2+ levels (Grohmann et al., 2003). Although increases in cAMP levels were also observed, it has been suggested that these increases were a secondary effect due to the increase in Ca2+ (Grohmann et al., 2003). Recently, several more distinct octopamine receptors that increase intracellular cAMP levels when activated have been isolated from Drosophila (Balfanz et al., 2005; Maqueira et al., 2005). Furthermore, comparison of the honey bee genome with the octopamine receptors found in Drosophila suggests that orthologs of these octopamine receptors also exist in bees (Evans and Maqueira, 2005). Activation of AmTYR1 receptors with tyramine leads to a reduction in intracellular cAMP levels (Blenau et al., 2000; Mustard et al., 2005) suggesting that octopamine and tyramine may have opposing effects on signaling via cAMP. AmOA1 and AmTYR1 are widely expressed in the honey bee brain and show overlapping patterns of expression (Blenau et al., 2000; Grohmann et al., 2003; Mustard et al., 2005), including the mushroom bodies and central complex, brain structures believed to play a role in locomotion (Martin et al., 1998; Strauss and Heisenberg, 1993). Although the pharmacological profiles of the honey bee AmOA1 and AmTYR1 receptors have not been extensively investigated, analysis of other invertebrate receptors suggests that mianserin acts as an antagonist at octopamine receptors (Bischof and Enan, 2004; Maqueira et al., 2005) while yohimbine functions as an antagonist at tyramine receptors (Ohta et al., 2003; Saudou et al., 1990; Vanden Broeck et al., 1995).
Honey bees have the advantage of exhibiting a number of different behaviors that can be examined in the laboratory and in the field, at the level of individuals or as a colony. Although previous studies have suggested roles for octopamine and tyramine in behavior, these experiments have either focused on bees in the complex natural environment of the colony (Barron et al., 2002; Harris and Woodring, 1992; Schulz et al., 2002; Schulz and Robinson, 1999; Wagener-Hulme et al., 1999) or have used restrained bees to examine learning and sensory perception (Menzel et al., 1999; Mercer and Menzel, 1982; Scheiner et al., 2002). Observation of freely moving honey bees in a simplified laboratory setting complements other studies by allowing for detailed analysis of the effects of octopamine or tyramine on readily quantifiable behaviors. Adult forager honey bees exhibit a number of different motor behaviors such as walking, grooming and fanning. Continuous observation of individual subjects allows for construction of behavioral “profiles” detailing how much time bees spend engaged in each behavior. This study examines changes in locomotor behavior induced by octopamine, tyramine, the octopamine receptor antagonist mianserin, or the tyramine receptor antagonist yohimbine.
Materials and Methods
Subjects
Honey bees used in this study were from the New World Carniolan population maintained at the Rothenbuhler Honey Bee Research Laboratory at Ohio State University. Adult worker honey bees were collected from indoor colonies kept on a 12/12 h light/dark cycle at 27 to 30 ºC. Individual bees were captured in small glass vials and placed at −20 ºC until motionless. Subjects were then placed in a short piece of drinking straw and restrained with a strip of tape placed between the head and thorax. The honey bees were then fed 18 μl of 2 M sucrose and left overnight at room temperature. After 24 hours, subjects were fed 9.0 μl of 2 M sucrose immediately prior to injection.
Treatment
Bees were treated with octopamine, tyramine, mianserin or yohimbine (Sigma Aldrich, St Louis, Mo.) over a range of concentrations made by serial dilutions in buffer (5 mM KCl, 10 mM NaH2PO4, pH 7.8). Concentrations ranged between 5 × 10−2 to 5 × 10−5 M for octopamine and tyramine. The high concentration (5 × 10−2 M) of the synthetic ligands appeared to increase mortality, so doses from 5 × 10−3 to 5 × 10−6 M were used for yohimbine and mianserin. Treatment consisted of 2 μl of buffer containing the indicated concentration of drug, or buffer alone as a control, injected under the cuticle between the second and third abdominal segments using a 10 μl syringe (Hamilton). To allow the treatment time to spread after injection, bees were kept in their harnesses until 5 min before the observation period was to begin. The first experiment was done with individuals receiving octopamine treatment. Analysis of these data suggested that significant effects of drug treatment were not seen until 30 min after injection. Therefore, for experiments with all other treatments we shifted our observations to 30–50 min after injection. Each subject was only used once.
Behavioral Observation
The observation arena was a standard 150 × 15 mm sterilized Petri dish (Fisher). Honey bees were released from their harnesses into the arena and allowed to acclimate to the arena for five minutes before observations began. A behavioral profile was determined for each subject by determining how much time each bee spent engaged in one of six mutually exclusive behaviors: walking, stopped, upside down, grooming, fanning or flying. Upside down behavior was seen when bees walking on the lid or side of the arena fell off onto their backs so that they were lying on their dorsal surface with their legs in the air. Any situation in which the bee used its legs to rub other parts of its body was considered grooming. Fanning consisted of stationary bees with raised abdomens that would rapidly beat their wings. Since the observation arenas are covered with a lid to prevent the subject from flying away, flying behavior consisted of short flying hops. The data was collected using The Observer (Noldus Information Technology) software in which each behavior is given a designated computer key that is pressed when the behavior is observed. The program then records the amount of time that passes before another key is pressed indicating the subject has shifted to a different behavior. All observations were done by one individual (BLF) and 10 to 14 individuals were observed for each treatment.
Data Analysis
The behavioral data were examined by breaking each observation up in to 5 min intervals. The amount of time spent engaged in each behavior during each interval was determined by The Observer program. Data were analyzed using the general linear modeling procedure in JMP (SAS Institute). We evaluated the effect of different drug dosages on the frequencies of different behaviors within each of the defined time intervals. The main significance test of interest was the interaction of behavior frequencies with dosage. A significant interaction indicates that the amount of time spent in each behavior within that time interval depends on drug dosage. Changes in the significance of the interaction terms across successive time intervals provide an indication of onset and offset of the drug effects.
If the interaction term was significant, post-hoc t-tests were performed to compare the time bees spent in each activity for each concentration of treatment with the time spent in that activity by buffer injected control subjects. A significant post-hoc test (p≤0.05) is indicated by asterisks within columns in figures. Furthermore, we separated flying behavior into a separate figure (Figs 1–4B) to show the results of post-hoc tests. Bees spent only a short amount of time flying. Thus, differences in flying due to treatment were not as visually obvious when plotted with other, more frequently observed behaviors
Figure 1.
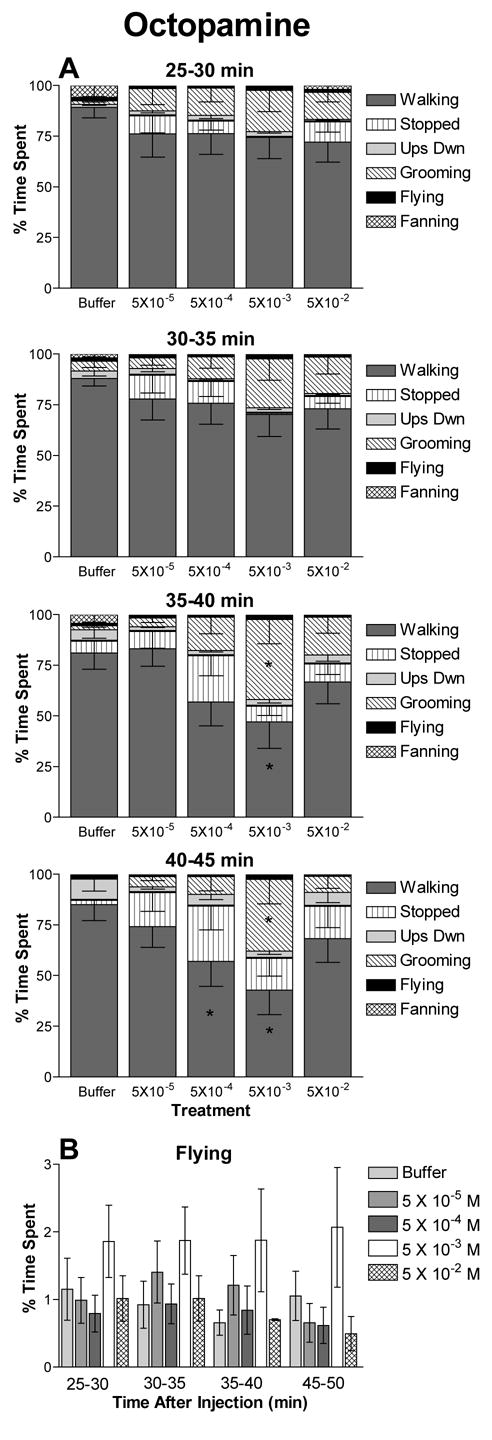
The effects of octopamine on honey bee behavioral profiles. The percent of time subjects spent engaged in each behavior is shown for treatment with each concentration of octopamine. Treatment consisted of injection with 2 μl of 5 × 10−2, 5 × 10−3, 5 × 10−4, 5 × 10−5 M octopamine or buffer alone. Data represent the average of 11 to 13 independent observations and error bars indicate the SEM. The interval of time post injection is indicated. A) Behavioral profiles are significantly different among bees given different treatments at the 35–40 and the 40–45 min intervals. B) Time spent flying versus time after injection for bees treated with octopamine. This is the same data included in the behavioral profiles in A shown on a separate graph to aid comparison. For intervals in which significant differences in behavioral profiles occurred, asterisks indicate significant differences in time spent in that behavior by bees treated with the indicated concentration of octopamine versus buffer controls (t test: *p ≤ 0.05).
Figure 4.
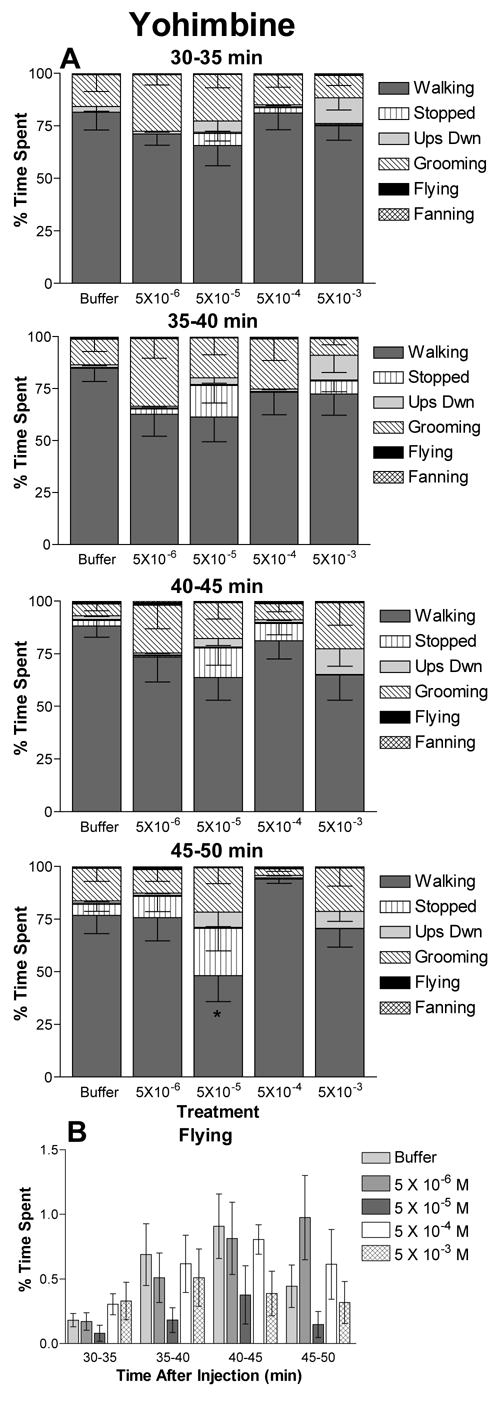
The effects of the tyramine receptor antagonist yohimbine on honey bee behavioral profiles. The percent of time subjects spent engaged in each behavior is shown for treatment with each concentration of yohimbine. Treatment consisted of injection with 2 μl of 5 × 10−3, 5 × 10−4, 5 × 10−5, 5 × 10−6 M yohimbine or buffer alone. Data represent the average of 12 to 14 independent observations and error bars indicate the SEM. The interval of time post injection is indicated. A) Behavioral profiles are significantly different among bees given different treatments at the 45–50 min interval. B) Time spent flying versus time after injection for bees treated with yohimbine. This is the same data included in the behavioral profiles in A shown on a separate graph to aid comparison. For intervals in which significant differences in behavioral profiles occurred, asterisks indicate significant differences in time spent in that behavior by bees treated with the indicated concentration of yohimbine versus buffer controls (t test: * p ≤ 0.05).
Results
In general, all of the drugs tested produced significant time and concentration dependent profiles. However, the patterns of the effects were specific to each drug.
Octopamine
Injection of octopamine into the hemolymph had strongest effects for intermediate (5 × 10−4 and 5 × 10−3 M) concentrations 35 to 45 min after injection (Figure 1). The amount of time bees spent engaged in each behavior did not differ across concentration, including buffer only control, for the 25–30 (F20,20 = 0.751, p = 0.77) and 30–35 (F20,20 = 0.953, p = 0.51) min post injection intervals. However, the trends evident at the early time intervals became significant at the later time intervals. Significant concentration dependent effects occurred at both the 35–40 (F20,20 = 2.44, p = 0.0006) and 40–45 min (F20,20 = 2.22, p = 0.0021) intervals. Bees treated with 5 × 10−3 M octopamine showed the largest changes, with a significant reduction in walking behavior in favor of time spent grooming. Bees treated with octopamine exhibited a general increase in the amount of time they spent stopped, however, these increases were not significantly different from controls. The relative time spent grooming or stopped differed between 5 × 10−3 and 5 × 10−4 M. At the former concentration, relatively more time was spent grooming. At the latter concentration, relatively more time was spent stopped. Subjects injected with 5 × 10−3 M octopamine also showed increases in time spent flying compared to buffer injected (control) bees at all time intervals (Fig 1B). However, post-hoc analysis indicated that these increases did not differ from control. Finally, injection of bees with either lower (5 × 10−5 M) or higher (5 × 10−2 M) concentrations of octopamine produced reductions in walking and increases in grooming and stopped behaviors, but these effects were relatively small compared to those seen for the intermediate concentrations.
Tyramine
Tyramine influenced the expression of behavior in a way that, in general, increased with concentration and time (Figure 2). Although the behavioral profiles were not different at the 30–35 (F20,20 = 1.19, p = 0.25) min interval, a significant interaction of time spent in each behavior with tyramine concentration was observed at the 35–40 (F20,20 = 2.42, p = 0.0007), 40–45 (F20,20 = 2.43, p = 0.0006) and 45–50 (F20,20 = 2.89, p = 0.0001) min intervals. Honey bees given tyramine showed a general trend to decrease time spent walking and increase time spent grooming, stopped or upside down compared to the control group. In the 30 to 40 min intervals, a decrease in walking occurred due to an increase in time spent grooming. The pattern changed in the 40 to 50 min range, when more time was spent stopped. The overall trend was for a reduction in walking and a concurrent increase in other behaviors with increasing tyramine concentration. Treatment with tyramine also reduced time engaged in flying for all concentrations after 35 min post injection (Figure 2B). However, differences in flying behavior were only significant at the 40–45 and 45–50 min intervals.
Figure 2.
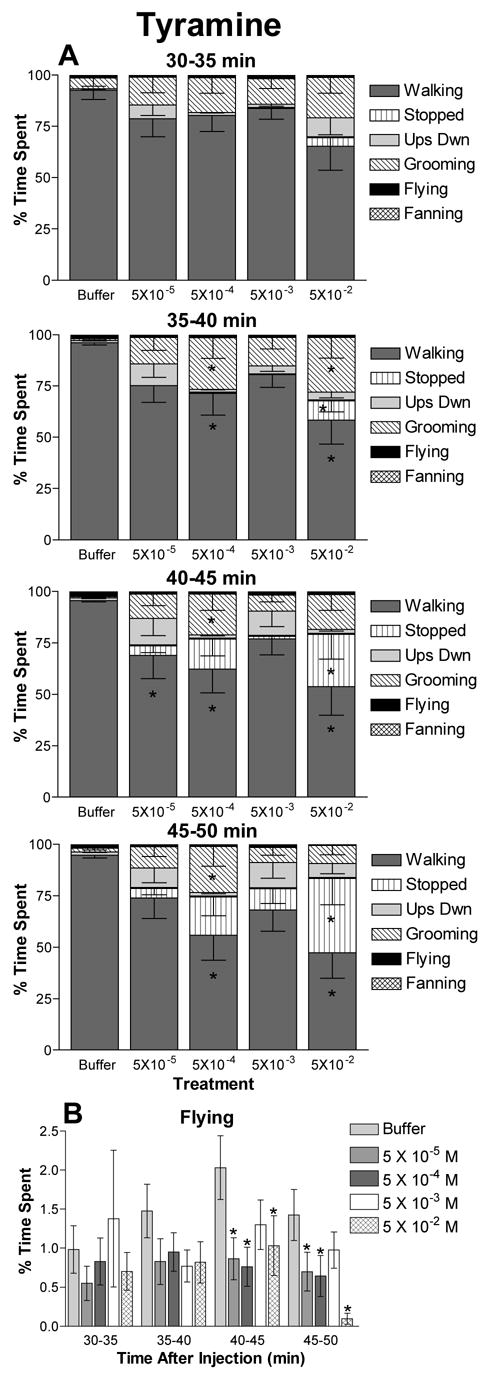
The effects of tyramine on honey bee behavioral profiles. The percent of time subjects spent engaged in each behavior is shown for treatment with each concentration of tyramine. Treatment consisted of injection with 2 μl of 5 × 10−2, 5 × 10−3, 5 × 10−4, 5 × 10−5 M tyramine or buffer alone. Data represent the average of 10 to 14 independent observations and error bars indicate the SEM. The interval of time post injection is indicated. A) Behavioral profiles are significantly different among bees given different treatments at the 35–40, 40–45 and the 45–50 min intervals. B) Time spent flying versus time after injection for bees treated with tyramine. This is the same data included in the behavioral profiles in A shown on a separate graph to aid comparison. For intervals in which significant differences in behavioral profiles occurred, asterisks indicate significant differences in time spent in that behavior by bees treated with the indicated concentration of tyramine versus buffer controls (t test: * p ≤ 0.05).
Mianserin
Treatment of worker bees with the putative octopamine receptor antagonist mianserin produced shifts in locomotor behavior at the lower concentrations and the earlier time intervals (Figure 3). The 30–35 min (F20,20 = 4.78, p = 0.0001) and 40–45 min (F20,20 = 2.54, p = 0.0004) intervals showed significant interaction of behavior with concentration; changes observed at the 35–40 min were barely not significant (F20,20 = 1.51, p = 0.07). The behavioral profiles for the 45–50 min interval (F20,20 = 0.871, p = 0.62) were not significantly different. Interestingly, the largest changes in behavior were seen in subjects given the lowest two doses (5 × 10−6 or 5 × 10−5 M). Early in the observation period (30–40 min), bees treated with 5 × 10−5 M mianserin spent significantly less time walking and more time grooming than control bees. At later intervals, these bees spent more time stopped. Injection with mianserin also affected flying behavior as treatment with the highest dose, 5 × 10−3 M, caused a reduction in time spent flying throughout the observation period (Fig 3B). However, post-hoc analysis determined that this reduction was only significant at the 30–35 min post injection interval.
Figure 3.
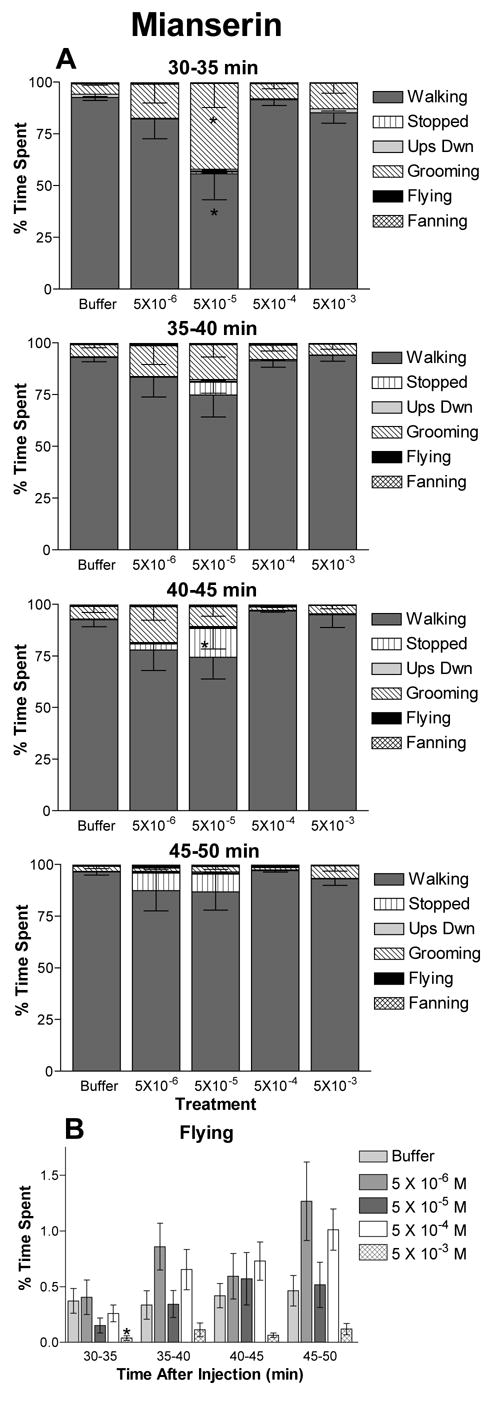
The effects of the octopamine receptor antagonist mianserin on honey bee behavioral profiles. The percent of time subjects spent engaged in each behavior is shown for treatment with each concentration of mianserin. Treatment consisted of injection with 2 μl of 5 × 10−3, 5 × 10−4, 5 × 10−5, 5 × 10−6 M mianserin or buffer alone. Data represent the average of 10 independent observations and error bars indicate the SEM. The interval of time post injection is indicated. A) Behavioral profiles are significantly different among bees given different treatments at the 30–35 and the 40–45 min intervals. B) Time spent flying versus time after injection for bees treated with mianserin. This is the same data included in the behavioral profiles in A shown on a separate graph to aid comparison. For intervals in which significant differences in behavioral profiles occurred, asterisks indicate significant differences in time spent in that behavior by bees treated with the indicated concentration of mianserin versus buffer controls (t test: * p ≤ 0.05).
Yohimbine
Similarly, the putative tyramine receptor antagonist yohimbine produced the largest alteration in behavioral profiles at the lower concentrations (Figure 4). In general, treatment with yohimbine caused small decreases in the amount of time bees spent walking and increases in time spent stopped, upside down or grooming. The 30–35 (F20,20 = 1.19, p = 0.25), 35–40 (F20,20 = 1.32, p = 0.16) and 40–45 (F20,20 = 1.22, p = 0.23) min intervals did not show significant differences for time spent in each behavior with yohimbine concentration. However, a significant change in behavioral profiles was observed for the 45–50 min interval (F20,20 = 2.89, p = 0.0001). The largest effect on behavior was on bees treated with 5 × 10−5 M yohimbine. These bees showed a significant reduction in time spent walking.
Discussion
These studies show that octopamine and tyramine affect the locomotor behavior of adult worker honey bees. Injection of any of the four treatments caused a shift in behavioral profile, although the observed effects were in general larger for subjects given the endogenous compounds tyramine or octopamine than for the synthetic antagonists. Injection of the biogenic amines octopamine, dopamine or serotonin into the hemolymph of cabbage looper moths, Trichoplusiani, has been shown to increase the levels of the injected compound significantly in the hemolymph, brain and thoracic ganglion within 30 min post injection (Linn et al., 1994). In honey bees, abdominal injection of biogenic amines or synthetic biogenic amine receptor ligands have been shown to effect sucrose response thresholds (Scheiner et al., 2002) and aggression (Robinson et al., 1999). Taken together, the results of these studies suggest that compounds injected into the hemolymph are able to reach targets in the central nervous system and influence behavior. All of the treatments caused at least a small increase in stopped behavior compared to controls. Although the increase in stopped behavior, which is rarely observed for control bees, may be indicative of the subjects feeling “sick”, this is probably not the case as the bees that spent the most time stopped were usually not the bees given the largest dose.
Rhythmic behaviors such as walking, grooming, and flying are controlled by central pattern generators (for reviews see Marder and Buchner, 2001; Marder, 2005). The basic patterns for these stereotyped repetitive movements are frequently generated in the thoracic ganglia. However, the rhythm can be modulated by sensory feedback (Büschges, 2005; Marder, 2005; Ridgel and Ritzmann, 2005). A number of studies have implicated octopamine in the modulation of invertebrate central pattern generators (Claassen and Kammer, 1986; Mulloney et al, 1987; Ridgel and Ritzmann, 2005; Stevenson and Kutsch, 1987). Unfortunately, in most of these studies the role of tyramine was not examined. Recent work in Drosophila larvae has shown that octopamine and tyramine act on neurons involved in pattern generation, and that it may be the relative levels of these two substances that are important for modulating behavior (Fox et al., 2006; Saraswati et al., 2004).
Grooming behavior increased, at least to some extent, in bees given any of the four treatments, implicating both biogenic amines in expression of grooming behavior. Similarly, Yellman et al. (1997) observed that application of octopamine, tyramine or dopamine to the nerve cord of decapitated Drosophila resulted in an increase in leg grooming behavior. Grooming takes place in all terrestrial species and is important for the maintenance of the outer surface of animals and for the removal of parasites (Sachs 1988; Spruijt et al., 1992). However, grooming is also observed in situations where it does not appear to be contributing to either of these functions. For example, in rodents elevated levels of grooming are observed in novel or stressful situations (Komorowska and Pellis, 2004; Pardon et al., 2004; van Erp et al., 1994). This “irrelevant” expression has lead to the description of grooming as a displacement activity that takes place when two motivational systems are activated leading to a state of uncertainty (Spruijt et al., 1992). In this context, the increase in grooming behavior observed after treatment may be due to activation (or inhibition) of multiple or conflicting pathways. Furthermore, grooming may also be associated with “dearousal” or a reduction in stress (Delius, 1988; Komorowska and Pellis, 2004; Spruijt et al., 1992). In invertebrates, octopamine has been associated with an increase in arousal (Corbet, 1991; Davenport and Evans, 1984; Orchard et al., 1981) and the increase in grooming may reflect an attempt to reduce this level of arousal.
Flying behavior was differentially affected by octopamine and tyramine. Although post-hoc analysis revealed time spent flying was not significantly different compared to controls, octopamine treatment generally increased flying behavior. On the other hand, bees given tyramine or high concentrations of mianserin showed decreases in time spent flying. Bees could not engage in extended bouts of flight in the observation arena, but exhibited short flying “hops”. Thus, our data do not reflect the amount of time subjects would have engaged in sustained flight, but are more likely to show that the bees were motivated to fly. Our results are consistent with other studies of the roles of octopamine in regulation of flying. Octopamine has been shown to play a number of important roles in insect flight including activation of the flight motor pattern (Stevenson and Kutsch, 1987) and regulation of glycolytic versus lipid pathways for generation of energy during flight (Candy, 1978; Mentel et al., 2003). Although high concentrations of mianserin reduced flying, in general mainserin had complex effects on flying behavior with some concentrations increasing and some decreasing time spent flying. This may be due to differences in affinities of this ligand for different octopamine receptor subtypes.
As well as working directly on central pattern generators, octopamine and tyramine may modulate sensory information used to determine in which activity to engage. Adult worker honey bees undergo a developmental shift in behavior. Young bees work at in-hive tasks such as taking care of the brood whereas older workers undertake tasks outside of the colony such as foraging for nectar or pollen (Winston, 1987). This shift from in-hive to outdoor tasks requires a change in the locomotor profiles for workers such as, for example, an increase in time spent flying versus walking. Interestingly, feeding octopamine to worker bees increases the number of young bees engaged in foraging whereas feeding tyramine leads to a reduction (Schulz and Robinson, 2001). Octopamine and tyramine appear to play roles in the modulation of sensory information as they affect the sucrose response threshold (Scheiner et al., 2002), odor perception (Kutsukake et al., 2000), vision (Erber and Kloppenburg, 1995), and mechanosensory information (Bräunig and Eder, 1998; Matheson, 1997; Widmer et al., 2005). This suggests that these substances may affect how incoming sensory information is perceived, thereby modifying the behavioral response. Grooming behavior in honey bees can be stimulated by the presence of parasites or particles on the cuticle (Land and Seeley, 2004; Pettis and Pankiw, 1998). If the injection of the drugs affected the processing of mechanosensory information, then this may also explain the increases in grooming behavior.
We have manipulated the octopamine and tyramine neurotransmitter or neuromodulatory pathways by injecting the native ligands or their putative antagonists. This is a necessary first step in establishing the role of these pathways in regulating locomotory behavior in the honey bee. However, this approach is complicated by several issues. First, although only one octopamine and one tyramine receptor have been cloned from honey bee, comparison of the honey bee genome with receptor genes from Drosophila suggest there are multiple subtypes of these receptors (Cazzamali et al., 2005; Maqueira et al., 2005). These receptors may have different affinities for their endogenous ligands and putative antagonists. Different receptor subtypes may also be coupled to distinct second messenger pathways such as cAMP or Ca2+ (Blenau and Baumann, 2001, 2003). Moreover, most biogenic amine receptors are activated by high concentrations of other amines (for examples see: Blenau et al., 2000; Reale et al., 1997; Rex and Komuniecki, 2002) making activation of other receptor types possible at high concentrations. Given that the injected ligands may be acting on multiple receptors thereby activating (or inhibiting) different behavioral pathways, it is not unexpected that a straightforward dose-response curve was not observed. Another complication is the lack of clear cut pharmacological tools such as receptor specific antagonists. For example, mianserin has been shown to act as a high affinity antagonist at octopamine receptors (Bischof and Enan, 2004; Maqueira et al., 2005). However, it may also act as an inverse agonist reducing the basal activity of some octopamine receptors (Maqueira et al., 2005). Furthermore, in honey bee brain homogenates, mianserin binds to a serotonin sensitive site (Blenau et al., 1995), although with lower affinity than at octopamine sensitive sites (Degen et al., 2000). A third issue complicating the interpretation of the results is that tyramine is the substrate for the synthesis of octopamine, therefore, increases in the level of tyramine could lead to increases in octopamine. Although it is possible that some of the effects observed for treatment with tyramine were due to an increase in octopamine, the fact that tyramine and octopamine produced distinct changes in behavior suggests that tyramine has an effect independent from that of octopamine.
This study complements behavioral studies on restrained honey bees or field work on the colony level by using continuous observation of freely moving adult bees to provide a sensitive assay by which to examine shifts in locomotor behavior. The data reported here suggest that octopamine and tyramine play a role in regulating motor function in honey bees. The injected compounds are able to reach targets in the brain and thoracic ganglion (Linn et al., 1994) on the time scale investigated here. Furthermore, octopamine and tyramine act at neuromuscular junctions (Kutsukake et al., 2000; Nagaya et al., 2002), suggesting that the injected ligands may also affect targets in the periphery. Further studies will be necessary to determine if these amines are acting via effects on central pattern generation, modulation of sensory processing, or if they are affecting higher order integrative pathways which determine in which behavior to engage. Given that there is evidence linking octopamine and tyramine with all of these processes, future work will need to examine specific regions of the nervous system and specific receptor subtypes to determine the roles of these signaling molecules in behavior.
Acknowledgments
The authors wish to thank Sue Cobey for maintaining the honey bee colonies. This work was supported by NIH (NIDA) grant DA017694 to JAM; NIH (NCRR) grant RR014166 to BHS; and by Ohio State University College of Biological Sciences Dean’s Undergraduate Research Awards to BLF.
References
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–260. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Balfanz S, Strünker T, Frings S, Baumann A. A family of octopamine receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. Journal of Neurochem. 2005;93:440–451. doi: 10.1111/j.1471-4159.2005.03034.x. [DOI] [PubMed] [Google Scholar]
- Barron AB, Schulz DJ, Robinson GE. Octopamine modulates responsiveness to foraging-related stimuli in honey bees (Apis mellifera) Journal of Comparative Physiology. 2002;188A:603–610. doi: 10.1007/s00359-002-0335-5. [DOI] [PubMed] [Google Scholar]
- Bischof LJ, Enan EE. Cloning, expression and functional analysis of an octopamine receptor from Periplaneta americana. Insect Biochemistry and Molecular Biology. 2004;34:511–521. doi: 10.1016/j.ibmb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Blenau W, May T, Erber J. Characterization of [H-3]LSD binding to a serotonin-sensitive site in honeybee (Apis mellifera) brain. Comparative Biochemistry and Physiology B. 1995;112:377–384. [Google Scholar]
- Blenau W, Balfanz S, Baumann A. Amtyr1: characterization of a gene from honeybee (Apis mellifera) brain encoding a functional tyramine receptor. Journal of Neurochemistry. 2000;74:900–908. doi: 10.1046/j.1471-4159.2000.0740900.x. [DOI] [PubMed] [Google Scholar]
- Blenau W, Baumann A. Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Archives of Insect Biochemistry and Physiology. 2001;48:13–38. doi: 10.1002/arch.1055. [DOI] [PubMed] [Google Scholar]
- Blenau W, Baumann A. Aminergic signal transduction in invertebrates: Focus on tyramine and octopamine receptors. Recent Research Developments in Neurochemistry. 2003;6:1–16. [Google Scholar]
- Bozic J, Woodring J. Variations of brain biogenic amines in mature honeybees and induction of recruitment behavior. Comparative Biochemistry and Physiology A. 1998;120:737–744. [Google Scholar]
- Bräunig P, Eder M. Locust dorsal unpaired median (DUM) neurones directly innervate and modulate hindleg proprioceptors. Journal of Experimental Biology. 1998;201:3333–3338. doi: 10.1242/jeb.201.24.3333. [DOI] [PubMed] [Google Scholar]
- Büschges A. Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. Journal of Neurophysiology. 2005;93:1127–1135. doi: 10.1152/jn.00615.2004. [DOI] [PubMed] [Google Scholar]
- Candy DJ. The regulation of locust flight muscle metabolism by octopamine and other compounds. Insect Biochemistry. 1978;8:177–181. [Google Scholar]
- Cazzamali G, Klaerke DA, Grimmelikhuijzen CJ. A new family of insect tyramine receptors. Biochemical and Biophysical Research Communications. 2005;338:1189–1196. doi: 10.1016/j.bbrc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Claassen DE, Kammer AE. Effects of octopamine, dopamine, and serotonin on production of flight motor output by thoracic ganglia of Manduca sexta. Journal of Neurobiology. 1986;17:1–14. doi: 10.1002/neu.480170102. [DOI] [PubMed] [Google Scholar]
- Corbet S. A fresh look at the arousal syndrome of insects. Advances in Insect Physiology. 1991;23:81–116. [Google Scholar]
- Davenport AP, Evans PD. Stress-induced changes in the octopamine levels of insect hemolymph. Insect Biochemistry. 1984;14:135–143. [Google Scholar]
- Degen J, Gewecke M, Roeder T. Octopamine receptors in the honey bee and locust nervous system: pharmacological similarities between homologous receptors of distantly related species. British Journal of Pharmacology. 2000;130:587–594. doi: 10.1038/sj.bjp.0703338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius JD. Preening and associated comfort behavior in birds. Annuals of the New York Academe of Science. 1988;525:40–55. doi: 10.1111/j.1749-6632.1988.tb38594.x. [DOI] [PubMed] [Google Scholar]
- Erber J, Kloppenburg P. The modulatory effects of serotonin and octopamine in the visual system of the honey bee (Apis mellifera L.) I. Behavioral analysis of the motion-sensitive antennal reflex. Journal of Comparative Physiology. 1995;176A:111–118. [Google Scholar]
- Evans PD, Maqueira B. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invertebrate Neuroscience. 2005;5:111–118. doi: 10.1007/s10158-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Fox LE, Soll DR, Wu CF. Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine Beta hydroxlyase mutation. Journal of Neuroscience. 2006;26:1486–1498. doi: 10.1523/JNEUROSCI.4749-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann L, Blenau W, Erber J, Ebert PR, Strünker T, Baumann A. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. Journal of Neurochemistry. 2003;86:725–735. doi: 10.1046/j.1471-4159.2003.01876.x. [DOI] [PubMed] [Google Scholar]
- Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learning & Memory. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- Harris JW, Woodring J. Effects of stress, age, season, and source colony on levels of octopamine, dopamine and serotonin in the honey bee (Apis mellifera L) brain. Journal of Insect Physiology. 1992;38:29–35. [Google Scholar]
- Komorowska J, Pellis SM. Regulatory mechanisms underlying novelty-induced grooming in the laboratory rat. Behavioral Processes. 2004;67:287–293. doi: 10.1016/j.beproc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kutsukake M, Komatsu A, Yamamoto D, Ishiwa-Chigusa S. A tyramine receptor gene mutation causes a defective olfactory behavior in Drosophila melanogaster. Gene. 2000;245:31–42. doi: 10.1016/s0378-1119(99)00569-7. [DOI] [PubMed] [Google Scholar]
- Land BB, Seeley TD. The grooming invitation dance of the honey bee. Ethology. 2004;110:1–10. [Google Scholar]
- Linn CE, Jr, Poole KR, Roelofs WL. Studies on biogenic amines and metabolites in nervous tissue and hemolymph of male cabbage looper moths - III. Fate of injected octopamine, 5-hydroxytryptamine and dopamine. Comparative Biochemistry and Physiology C. 1994;108:99–106. [Google Scholar]
- Maqueira B, Chatwin H, Evans PD. Identification and characterization of a novel family of Drosophila beta-adrenergic-like octopamine G-protein coupled receptors. Journal of Neurochemistry. 2005;94:547–560. doi: 10.1111/j.1471-4159.2005.03251.x. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Current Biology. 2001;11:R986–996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Current Biology. 2005;15:R685–699. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Martin JR, Ernst R, Heisenberg M. Mushroom bodies suppress locomotor activity in Drosophila melanogaster. Learning & Memory. 1998;5:179–191. [PMC free article] [PubMed] [Google Scholar]
- Matheson T. Octopamine modulates the responses and presynaptic inhibition of proprioceptive sensory neurones in the locust Schistocerca gregaria. Journal of Experimental Biology. 1997;200:1317–1325. doi: 10.1242/jeb.200.9.1317. [DOI] [PubMed] [Google Scholar]
- Mentel T, Duch C, Stypa H, Wegener G, Müller U, Pflüger HJ. Central modulatory neurons control fuel selection in flight muscle of migratory locust. Journal of Neuroscience. 2003;23:1109–1113. doi: 10.1523/JNEUROSCI.23-04-01109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R, Heyne A, Kinzel C, Gerber B, Fiala A. Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behavioral Neuroscience. 1999;113:744–754. [PubMed] [Google Scholar]
- Mercer AR, Menzel R. The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honeybee Apis mellifera. Journal of Comparative Physiology. 1982;145A:363–368. [Google Scholar]
- Mulloney B, Acevedo LD, Bradbury AG. Modulation of the crayfish swimmeret rhythm by octopamine and the neuropeptide proctolin. Journal of Neurophysiology. 1987;58:584–597. doi: 10.1152/jn.1987.58.3.584. [DOI] [PubMed] [Google Scholar]
- Mustard JA, Kurshan PT, Hamilton IS, Blenau W, Mercer AR. Developmental expression of a tyramine receptor gene in the brain of the honey bee, Apis mellifera. Journal of Comparative Neurology. 2005;483:66–75. doi: 10.1002/cne.20420. [DOI] [PubMed] [Google Scholar]
- Nagaya Y, Kutsukake M, Chigusa SI, Komatsu A. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neuroscience Letters. 2002;329:324–328. doi: 10.1016/s0304-3940(02)00596-7. [DOI] [PubMed] [Google Scholar]
- Ohta H, Utsumi T, Ozoe Y. B96Bom encodes a Bombyx mori tyramine receptor negatively coupled to adenylate cyclase. Insect Molecular Biology. 2003;12:217–223. doi: 10.1046/j.1365-2583.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- Orchard I, Loughton BG, Webb RA. Octopamine and short-term hyperlipaemia in the locust. General and Comparative Endocrinology. 1981;45:175–180. doi: 10.1016/0016-6480(81)90102-7. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Kendall DA, Perez-Diaz F, Duxon MS, Marsden CA. Repeated sensory contact with aggressive mice rapidly leads to an anticipatory increase in core body temperature and physical activity that precedes the onset of aversive responding. European Journal of Neuroscience. 2004;20:1033–1050. doi: 10.1111/j.1460-9568.2004.03549.x. [DOI] [PubMed] [Google Scholar]
- Pettis JS, Pankiw T. Grooming behavior by Apis mellifera L. in the presence of Acarapis woodi (Rennie) (Acari: Tarsonemidae) Apidologie. 1998;29:241–253. [Google Scholar]
- Reale V, Hannan F, Midgley JM, Evans PD. The expression of a cloned Drosophila octopamine/tyramine receptor in Xenopus oocytes. Brain Research. 1997;769:309–320. doi: 10.1016/s0006-8993(97)00723-3. [DOI] [PubMed] [Google Scholar]
- Rex E, Komuniecki RW. Characterization of a tyramine receptor from Caenorhabditis elegans. Journal of Neurochemistry. 2002;82:1352–1359. doi: 10.1046/j.1471-4159.2002.01065.x. [DOI] [PubMed] [Google Scholar]
- Ridgel AL, Ritzmann RE. Effects of neck and circumoesophageal connective lesions on posture and locomotion in the cockroach. Journal of Comparative Physiology. 2005;191A:559–573. doi: 10.1007/s00359-005-0621-0. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Heuser LM, Le Conte Y, Lenquette F, Hollingworth RM. Neurochemicals aid bee nestmate recognition. Nature. 1999;399:534–535. [Google Scholar]
- Sachs BD. The development of grooming and its expression in adult animals. Annuals of the New York Academe of Science. 1988;525:1–17. doi: 10.1111/j.1749-6632.1988.tb38591.x. [DOI] [PubMed] [Google Scholar]
- Saraswati S, Fox LE, Soll DR, Wu CF. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. Journal of Neurobiology. 2004;58:425–441. doi: 10.1002/neu.10298. [DOI] [PubMed] [Google Scholar]
- Saudou F, Amlaiky N, Plassat JL, Borrelli E, Hen R. Cloning and characterization of a Drosophila tyramine receptor. EMBO Journal. 1990;9:3611–3617. doi: 10.1002/j.1460-2075.1990.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner R, Plückhahn S, Öney B, Blenau W, Erber J. Behavioural pharmacology of octopamine, tyramine and dopamine in honey bees. Behavioural Brain Research. 2002;136:545–553. doi: 10.1016/s0166-4328(02)00205-x. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Robinson GE. Biogenic amines and division of labor in honey bee colonies: behaviorally related changes in the antennal lobes and age-related changes in the mushroom bodies. Journal of Comparative Physiology. 1999;184A:481–488. doi: 10.1007/s003590050348. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Robinson GE. Octopamine influences division of labor in honey bee colonies. Journal of Comparative Physiology. 2001;187A:53–61. doi: 10.1007/s003590000177. [DOI] [PubMed] [Google Scholar]
- Schulz DJ, Barron AB, Robinson GE. A role for octopamine in honey bee division of labor. Brain and Behavioral Evolution. 2002;60:350–359. doi: 10.1159/000067788. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. Journal of Neuroscience. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiology Reviews. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Stevenson PA, Kutsch W. A reconsideration of the central pattern generator concept for locust flight. Journal of Comparative Physiology. 1987;161A:115–129. [Google Scholar]
- Strauss R, Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. Journal of Neuroscience. 1993;13:1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp AM, Kruk MR, Meelis W, Willekens-Bramer DC. Effect of environmental stressors on time course, variability and form of self-grooming in the rat: handling, social contact, defeat, novelty, restraint and fur moistening. Behavioural Brain Research. 1994;65:47–55. doi: 10.1016/0166-4328(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Vanden Broeck J, Vulsteke V, Huybrechts R, De Loof A. Characterization of a cloned locust tyramine receptor cDNA by functional expression in permanently transformed Drosophila S2 cells. Journal of Neurochemistry. 1995;64:2387–2395. doi: 10.1046/j.1471-4159.1995.64062387.x. [DOI] [PubMed] [Google Scholar]
- Wagener-Hulme C, Kuehn JC, Schulz DJ, Robinson GE. Biogenic amines and division of labor in honey bee colonies. Journal of Comparative Physiology. 1999;184A:471–479. doi: 10.1007/s003590050347. [DOI] [PubMed] [Google Scholar]
- Widmer A, Hoger U, Meisner S, French AS, Torkkeli PH. Spider peripheral mechanosensory neurons are directly innervated and modulated by octopaminergic efferents. Journal of Neuroscience. 2005;25:1588–1598. doi: 10.1523/JNEUROSCI.4505-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston M. The biology of the honey bee. Harvard University Press; Cambridge, MA: 1987. [Google Scholar]
- Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proceedings of the National Academy of Science USA. 1997;94:4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]


