Abstract
Ethanol consumption produces characteristic behavioral states in animals that include sedation, disorientation, and disruption of motor function. Using individual honey bees, we assessed the effects of ethanol ingestion on motor function via continuous observations of their behavior. Consumption of 1 M sucrose solutions containing a range of ethanol doses lead to hemolymph ethanol levels of approximately 40 to 100 mM. Using ethanol doses in this range, we observed time and dose-dependent effects of ethanol on the percent of time our subjects spent walking, stopped, or upside down, and on the duration and frequency of bouts of behavior. The effects on grooming and flying behavior were more complex. Behavioral recovery from ethanol treatment was both time and ethanol dose dependent, occurring between 12 and 24 hr post-ingestion for low doses and at 24 to 48 hours for higher doses. Furthermore, the amount of ethanol measured in honey bee hemolymph appeared to correlate with recovery. We predict that the honey bee will prove to be an excellent model system for studying the influence of ethanol on the neural mechanisms underlying behavior.
Keywords: alcohol, central pattern generator, invertebrate, grooming, locomotion
Introduction
Alcohol abuse and alcoholism are worldwide health problems with serious medical and social ramifications. In humans and other mammals, acute consumption of ethanol causes a dose-dependent change in motor function that produces behavioral effects such as hyperactivity, loss of coordination and sedation (for a review see Phillips and Shen, 1996). Using invertebrate model systems for the study of ethanol’s effects on the nervous system conveys several advantages, especially because many cellular mechanisms appear to be conserved across animal phyla (Wolf and Heberlein, 2003). In particular, studies using locomotor assays have extended what is known with respect to the molecular targets of ethanol in the central nervous system (Davies, et al., 2003; Moore, et al., 1998; Morgan and Sedensky, 1995; Scholz, et al., 2005; Wolf, et al., 2002). Using the fruit fly, Drosophila melanogaster, and the nematode, Caenorhabditis elegans, these studies have elegantly shown that ethanol interacts with calcium-activated potassium channels (Davies, et al., 2003), GABAB receptors (Dzitoyeva, et al., 2003), and signaling via dopamine (Bainton, et al., 2000) and cAMP (Moore, et al., 1998).
The honey bee (Apis mellifera) has also been used extensively to study molecular and neural mechanisms underlying learning and behavior (Giurfa, 2003; Menzel, 2001; Robinson, et al., 2005). Honey bees are excellent at learning visual and olfactory tasks; moreover, they exhibit complex social behaviors such as food sharing and division of labor (Seeley, 1985, 1995; Winston, 1987). In contrast to studies using Drosophila and C. elegans where ethanol is delivered to experimental subjects as a vapor or by injection (Drosophila) or via the media on which the animal lives (C. elegans), honey bees will readily consume ethanol (Abramson, et al., 2000). Furthermore, the larger size of honey bees makes it easier to observe subtle changes in behavioral state associated with ethanol consumption. We anticipate, therefore, that the use of the honey bee as a model for studying ethanol is likely both to enhance current studies of ethanol’s effects on motor function and to lead to a greater understanding of ethanol’s influence on learning and social interactions.
This series of experiments was designed to measure the extent to which ethanol affects the detailed pattern of motor behavior in honey bees in a dose and time-dependent manner. By observing the behavior of individual honey bees continuously for 40 min, we show that acute ethanol ingestion has significant effects on time spent in walking, grooming, and flying behavior as well as potentially disrupting the righting reflex that restores posture. Furthermore, measurement of ethanol concentration in the hemolymph (blood) revealed that ethanol affected honey bee behavior at doses similar to those that cause intoxication in humans, Drosophila and C. elegans (Davies, et al., 2003; Moore, et al., 1998; Singh and Heberlein, 2000). Also, we observed that recovery from consumption of ethanol was dose and time-dependent and predict that changes in behavior correlate with the reduction of hemolymph ethanol levels.
Materials and Methods
Subjects
European worker honey bees (Apis mellifera) were selected from an indoor colony maintained at 25–30 °C on a 12 h/12 h light/dark cycle. Individuals were collected in small glass vials and cooled at −20 ºC for 1–5 minutes. When a subject became inactive, it was removed from its vial, placed into a plastic restraining harness. Bees were fed 18 μl of 1.8 M sucrose solution and held for 24 h at room temperature before treatment with ethanol.
Observations
Individual subjects were placed directly into 150 x 15 mm Petri dishes (Fisher) that were used as observation arenas. Preliminary observations determined that the behavior of honey bees placed into the arena fell into one of six mutually exclusive categories: 1) walking; 2) stopped; 3) upside down (when the subject lies on its back with its legs in the air); 4) fanning (stopped behavior accompanied by vigorous wing beating); 5) flying; and 6) grooming. The time spent engaged in each behavioral state was recorded by the same person for all experiments (ISM) using The Observer software (Noldus Information Technology, Version 2.0 and 5.0). The Observer software allowed us to determine the frequency and duration of bouts of behavior as well as the time individuals spent exhibiting each behavior. Each subject was only observed at one specific time point. In the first set of experiments, twenty-five of the observations were made using the Observer 2.0; because this event recorder could not record events shorter than 1 s in duration, we were unable to record accurately the bout duration of behavioral events, such as flying events, that were often shorter than 0.5 s.
Onset of the effects of ethanol
To establish both the time of onset and the dose-dependency of ethanol induced changes in behavior, individuals were fed 9 μl of 1.0 M sucrose solution containing either 0, 5, 10, 25, 50, or 75% ethanol. Only honey bees that readily consumed the entire dose in a short period of time (under 2 min) were used in experiments. The sucrose concentration was the same for each ethanol treatment to ensure that observed behavioral effects were the result of ethanol dose and not due to sugar ingestion. Immediately after consuming one of the treatment solutions described above, a single subject was placed into the observation arena and observed continuously for 40 min (NTotal = 5 per treatment).
Time course of the effects of ethanol
To investigate the time course of the effects of ethanol and establish the time necessary for recovery, behavioral observations were done 0.5, 2, 4, 6, 12, 24 and 48 h after ingestion of ethanol. Each honey bee was fed 9 μl of one of five 1.0 M sucrose solutions containing different concentrations of ethanol (0, 5, 10, 25 or 50%) and was kept in its restraining harness until 10 min before the designated time point for observation. At that time, the bee was removed from its harness, placed into the observation arena for a 10 min acclimatization period and then observed for 10 min. The 48 h test subjects were fed 18 μl of 1.8 M sucrose solution 24 h after ethanol treatment to prevent them from starving.
Measurement of hemolymph ethanol levels
Ethanol levels in the hemolymph were measured to investigate if ethanol accumulation correlated with behavior. Honey bees were fed 9 μl of 1.0 M sucrose solution containing either 0, 5, 10, 25 or 50% ethanol. Hemolymph samples were taken 0.5, 6, 12, 24 or 48 h after consumption of ethanol. The 48 h test subjects were fed 18 μl of 1.8 M sucrose solution at 24 h after ethanol ingestion. 1μl of hemolymph was taken from the dorsal abdomen using a 1μl volumetric capillary tube (Drummond Scientific). Each bee was only sampled once. Samples were mixed with 19 μl of buffer solution (50 mM Tris-HCl, pH 8.0) and stored at −20 ºC. Samples were analyzed in duplicate using an endpoint ethanol assay (Diagnostic Chemicals Ltd, Oxford, CT USA) that uses alcohol dehydrogenase (ADH) to convert NAD and ethanol to NADH and acetylaldehyde. The change in absorbance at λ = 340 nm due to the reduction of NAD to NADH+ was determined. The absorbances of hemolymph samples from bees fed sucrose alone were used as the baseline values and were subtracted from the values for subjects consuming ethanol.
Mortality
To analyze the potentially toxic effects of ethanol consumption, mortality rates were investigated. Individual honey bees were collected and harnessed as described above. As in the previous experiments, each subject was fed 9 μl of 1.0 M sucrose solution containing either 0, 5, 10, 25 or 50% ethanol 24 h after being harnessed. At time points corresponding to the 10 min behavioral time course observations (0.5, 6, 12, 24 or 48 h), each subject was determined to be alive or dead. The 48 h bees were fed 24 h post-ethanol ingestion as in the behavioral studies.
Statistical Analysis
Statistical comparisons for treatments and intervals for each behavioral experiment were calculated using a repeated-measures GLM, 2-way ANOVA, or by Poisson regression; interaction terms and main effects in the models were reported only when significant. Multiple comparisons (MC) were calculated either as least-squares differences or as Dunnett’s MCs. Where appropriate, Bonferroni adjustments for experiment-wise error rate (EER) were applied and adjusted P-values reported. To control for EER, comparisons were made directly with the control unless otherwise stated. MCs were reported only when a main effect was significant.
Results
Behavioral effects and onset of inebriation are dose-dependent
Using individual worker honey bees, we observed that ethanol treatment produced dose-dependent effects for all the behaviors we recorded except fanning (Figure 1). We also observed that the amount of time subjects spent walking, stopped or upside down changed over the duration of the 40 min interval for all treatments (Figure 1A, B, C). Fanning was rarely observed and, in general, was exhibited less than 1% of the time. We used a 5 min interval to examine changes in the time spent in each behavior because it was the lowest interval over which there was not a change within the interval for any of the behaviors observed. In general, the amount of time subjects spent walking in each 5 min interval decreased (main effect of interval, repeated-measures general linear model (GLM), F1,271 = 63.9, P < 0.001). Furthermore, the amount of decrease in walking behavior was dose-dependent (ethanol treatment main-effect, repeated-measures GLM, F5,271 =45.5, P < 0.001). The onset of inebriation was also dose-dependent (Figure 1A). Onset was characterized as the 5 min interval during which the amount of time an ethanol treated subject spent walking was significantly different from the control. Onset occurred at 5–10 min for the 75% (P < 0.001) and 50% (P = 0.002) groups; at 10–15 min for the 25% group (P = 0.004), and at 20–25 min for the 10% group (P < 0.001). Bees treated with 5% ethanol did not show significant differences in walking compared to control bees even during the last interval (35–40 min, P = 0.068). (Multiple post-hoc comparisons were limited to comparisons of the control with each ethanol treatment because of the EER produced by making many comparisons). The amount of time honey bees spent upside down generally increased over the 40 min period (Figure 1B; interval main effect GLM, F1,271=7.65, P = 0.006). Time spent upside down was also dose-dependent (ethanol main effect GLM, F1,271=22.7, P < 0.001), but MC revealed that only the 50 and 75% ethanol treatment groups were significantly different from the control (MC, 50 and 75% both P < 0.001). The percent time stopped also increased over the 40 min observation period for all treatment groups (Figure 1C; interval main effect GLM, F1,271=22.7, P < 0.001). The amount of time spent stopped was dose-dependent (ethanol main effect GLM, F5,271=2.58, P = 0.026), however, MC revealed that only the 25% ethanol treatment was different from the control (MC, P = 0.015).
Figure 1.
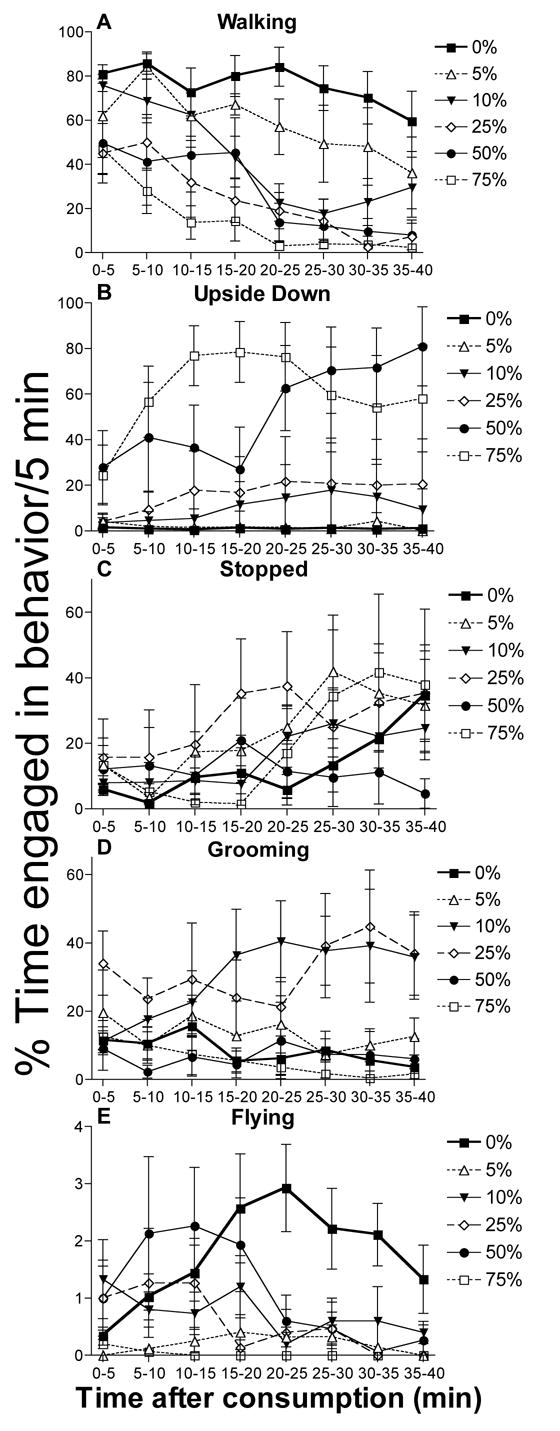
Ethanol affects honey bee behavior in a time and dose-dependent manner. The average percent time honey bees in each treatment group spent engaged in each behavior for a 5 min interval is shown +/− SEM (N = 5 per treatment). (A) Walking behavior, an indicator of ethanol inebriation, generally decreased over the interval (P < 0.001), and showed a dose-dependent response (P < 0.001). The onset of inebriation occurred earlier for higher ethanol doses. (B) Time spent upside down generally increased over the interval for all treatments (P < 0.001); but only the 50 and 75% ethanol treatments were significantly different from the control (P < 0.001). (C) Duration of time when the subject did not move (stopped) was dependent on ethanol treatment (P = 0.026) and increased over the 40 min period (P < 0.001). (D) Grooming behavior was greatest for the 10 and 25% ethanol treatments when compared to the control (P < 0.001). (E) Flying behavior was significantly lower (P ≤ 0.001) compared to the control for all treatments except the 50% ethanol group.
Ethanol ingestion affected grooming (ethanol treatment main effect GLM, F5,271=21.1, P < 0.001) and flying behavior (main effect GLM, F5,271=10.8, P < 0.001) in a dose-dependent manner (Figure 1D, 1E), but neither behavior changed substantially as a function of time interval (interval main effect GLM, grooming: F1,271= 265, P = 0.751; flying: F1,271=12.6, P = 0.406). Interestingly, both grooming and flying behavior showed non-linear dose-dependent effects when compared to the control group. In particular, an increase in grooming behavior was observed only for subjects in the 10 and 25% ethanol groups relative to the control (MC, 10 and 25% both P < 0.001). For flying behavior, subjects given 5, 10, 25 or 75% ethanol exhibited significantly less flying activity than the control group (MC, all P ≤ 0.001), whereas the 50% ethanol treated subjects did not differ from the control (MC, P = 0.071).
We also analyzed the pattern of behavior over the 40 min interval by examining the mean duration and frequency of the bouts of walking, stopped, upside down and grooming behavior (Figure 2). Bouts of flying behavior often had durations of less than one second, which was shorter than the resolution of the Observer program (version 2). This prevented us from analyzing bout structure for flying. Bout duration and frequency varied substantially for honey bees in each treatment group when compared to the bout structure of the control subjects (Figure 2). In general, bees given 5% ethanol differed from the control group only in that they had significantly longer bouts of walking activity and more frequent bouts of stopped behavior. Honey bees receiving 10, 25, 50, or 75 % ethanol treatments had significantly shorter and less frequent bouts of walking activity, stopped more frequently and had longer bouts of upside down behavior than control bees. The 10 and 25% ethanol treatment groups exhibited both longer and more frequent bouts of grooming behavior when compared to the control.
Figure 2.
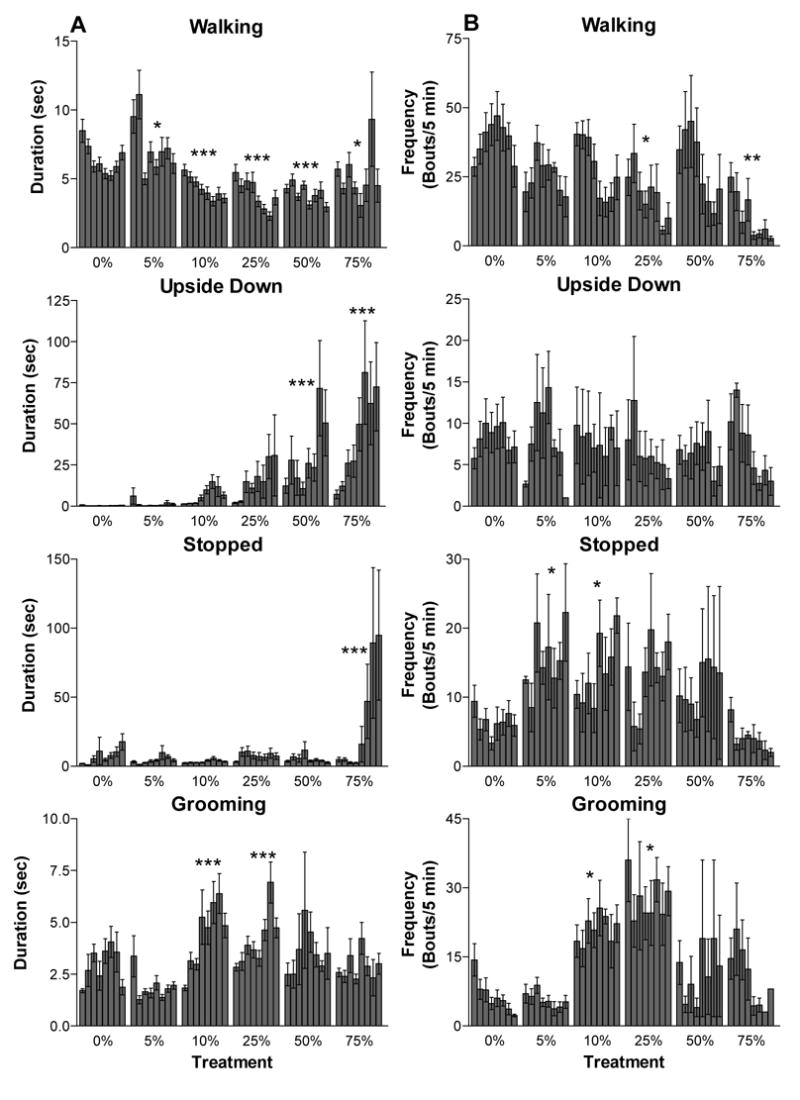
Duration and frequency of bouts of behavior during the first 40 min after ethanol consumption. (A) The mean duration of a bout of behavior during each 5 min interval ± SEM is shown for walking, upside down, stopped and grooming behavior. (B) Mean frequency (number of times a bout of the behavior was observed) of bouts of walking, upside down, stopped and grooming for each 5 min interval. Mean bout duration and frequency for the entire 40 min observation were compared to the control (0%) for subjects given each ethanol treatment for each behavior. Bout durations were compared with a Dunnett’s test and frequencies were compared with a least-squares contrast. Asterisks (*) indicate significant P-values (* p < 0.05, ** p < 0.01 *** p < 0.005).
When the entire 40 min interval was examined in greater detail, bout duration of walking behavior decreased per 5 min interval for all groups (main effect GLM, F7,271= 2.28, P = 0.029). Mean bout duration also decreased as a function of ethanol concentration (main effect GLM, F5,271=25.6, P < 0.001). The frequency of bouts of walking in a given 5 min interval depended both on ethanol concentration and on the time interval (Pois. reg. χ62 = 17.4, P = 0.008; Figure 2), as bees given ethanol treatments of 10% or greater exhibited a decline in the frequency of walking bouts in the last 20 min of the observation period. The duration and frequency of bouts of stopped behavior depended only on the ethanol treatment and did not change over the 40 min observation (duration: main effect GLM, F5,271=7.10, P < 0.001; freq.: Pois. reg. χ52 = 12.5, P = 0.028). Mean bout duration of upside down behavior increased as a function of the concentration of ethanol ingested (main effect GLM, F5,271=21.1, P < 0.001). For honey bees receiving ethanol doses of 10% or more, mean bout duration also increased as a function of time interval, such that bees had the longest bouts of upside down behavior at the latest time intervals (main effect GLM, F7,271=2.95, P = 0.005). While bout duration increased over the 40 min interval, the frequency of bouts of upside down behavior generally decreased over the interval (Pois. reg. χ12 = 11.3, P = 0.014); however, the frequency was not significantly different between the treatment groups (Pois. reg. χ62 = 2.44, P = 0.875). Grooming behavior bout duration was affected by the ethanol treatments honey bees received (main effect GLM, F5,271=8.73, P < 0.001), but it did not vary over the time interval (main effect GLM, F7,271=0.57, P = 0.777). The frequency of bouts of grooming was dependent upon both the ethanol dose bees consumed and the time interval (interaction: Pois. reg. χ62 = 12.1, P = 0.05); honey bees receiving the intermediate concentrations of ethanol (10 and 25%) had both the most frequent and longest bouts of grooming behavior.
Recovery from ethanol inebriation is dose and time-dependent
To assess recovery from ethanol consumption, we examined the same behavioral classes assayed above over a 10 min interval at one of seven time points (0.5, 2, 4, 6, 12, 24 or 48 h) after ethanol ingestion (Figure 3). For each time point, the behavior of honey bees consuming ethanol was compared to that of the control group (Dunnett’s MC); MCs were limited to comparison with the control within each time point to control for EER. Recovery was both time and dose-dependent (Figure 3). For walking, upside down, and flying behaviors, complete recovery, such that the time subjects spent in each behavior was the same as the controls, depended both on the time point sampled and the ethanol dose received (ethanol by time interaction GLM: walk: F24,400=2.23, P < 0.001; upside down: F24,400=2.51, P < 0.001; flying: F24,400=1.73, P = 0.019). Thus, via comparison with control subjects, we concluded that recovery from ethanol inebriation occurred by 24 h for honey bees receiving 5 or 10% treatments and by 48 h for subjects given 25 or 50% ethanol (Figure 3). Relative to the control, flying behavior was depressed for subjects given ethanol at many time points, although there was a substantial amount of variation between individuals (Figure 3). The amount of time spent in stopped behavior was a function of the time post-ingestion (main effect: F6,400=2.71, P = 0.013) and ethanol treatment (main effect: F6,400=4.05, P = 0.003) independently. Because it showed high variability within treatment groups, grooming behavior did not significantly differ among treatments and was dependent only on the time points sampled after consumption (main effect: F4,400=4.03, P = 0.001).
Figure 3.
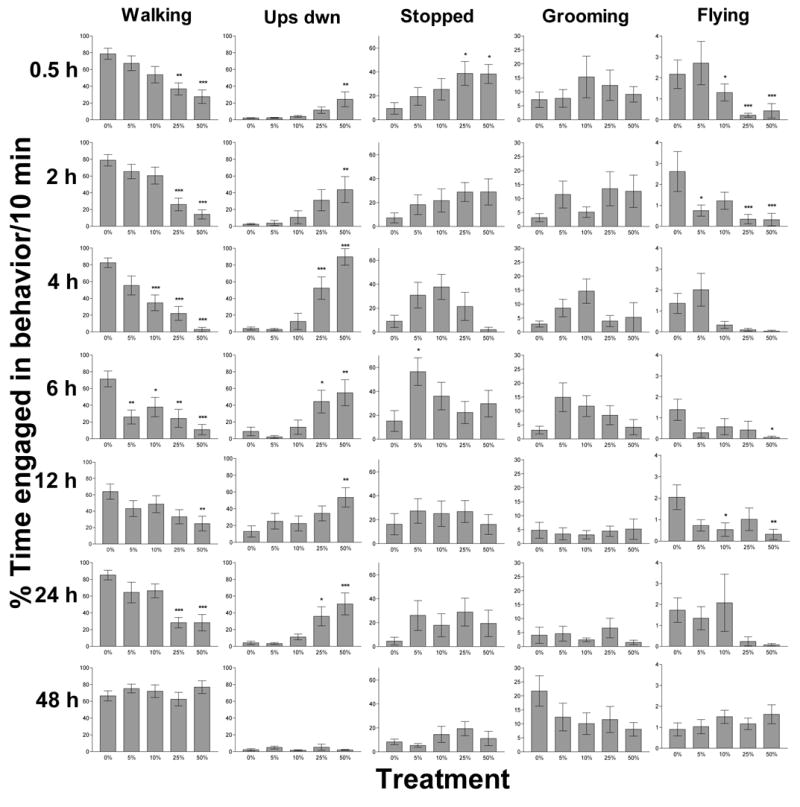
Recovery time from ethanol inebriation was dose-dependent (P < 0.001) and occurred for all treatments by 48 h post-ingestion. To assess recovery, we compared time spent walking for subjects in each ethanol treatment to the control group (0%). Although the amount of time engaged in walking is slightly lower than that for control, subjects given 5 or 10% ethanol appear to recover between 12–24 h after ethanol ingestion. In contrast, the subjects that ingested 25 or 50% ethanol required 24 to 48 h to recover. Asterisks (*) indicate significant P-values for Dunnett’s multiple comparisons of each ethanol treatment against the control (* p < 0.05; ** p < 0.01; *** p < 0.001). Bars represent the mean time subjects spent in the indicated behavior +/− SEM. N = 10 per treatment for the 0.5, 2, 4, 6, and 24 h time points and N = 15 per treatment for the 12 and 48 h time points.
Levels of ethanol in the hemolymph are dose and time-dependent
Hemolymph ethanol levels depended on both the dose of ethanol consumed and the time after consumption (Figure 4; 2-way analysis of variance (ANOVA), time point main effect: F4,125 = 19.0, P < 0.001; ethanol treatment main effect, F3,125 = 19.3, P < 0.001). The amount of ethanol observed in the hemolymph was dose dependent such that honey bees that ingested higher concentrations of ethanol also had concomitantly greater amounts of ethanol present in the hemolymph. For example, 30 min after consumption bees given 5% ethanol had an average of about 40 mM ethanol in their hemolymph, this level of ethanol was maintained for around 6 hours and then decreased to near baseline levels by 12 hours (Figure 4). On the other hand, at 30 min honey bees given the same volume of solution containing 50% ethanol had approximately 100 mM ethanol in their hemolymph, and at 24 hours hemolymph ethanol levels for these bees were still high (87 mM). It wasn’t until between 24 and 48 hours post-ingestion that hemolymph ethanol levels returned to baseline for bees treated with the 50% ethanol dose (Figure 4). MCs revealed that subjects in the 10 and 25% treatment groups were the only two groups that did not have significantly different hemolymph ethanol levels (MC, P = 0.923). The level of ethanol in the hemolymph also decreased over time. Comparison of hemolymph ethanol levels at the 30 min time point to the 6 other time points revealed that the 30 min level was not significantly different from the time points measured after 6 or 12 h, but it was significantly greater than the 24 or 48 h time points (Dunnett’s MC: 24 h: P < 0.001, 48 h: P < 0.001).
Figure 4.
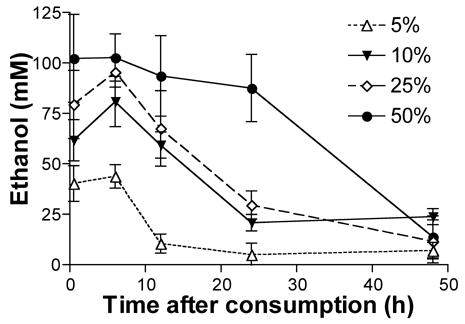
Ethanol in honey bee hemolymph decreases as a function of time post ethanol ingestion. The concentration of the ethanol in the hemolymph of subjects that consumed 1.0 M sucrose solution containing 5, 10, 25, or 50% ethanol was measured at different times (0.5, 6, 12, 24 or 48 h) post-ingestion. Ethanol levels were significantly lower for the 24 and 48 h time points when compared with the 0.5 h time point for all treatments. All treatments, except the 10 and 25%, had significantly different ethanol concentrations (P ≤ 0.001). Each point represents the mean +/− SEM. At 0.5 hr: N5% = 7, N10% = 8, N25% = 7, N50% = 8, Ncontrol = 7; at 6 hr: N5% = 6, N10% = 5, N25% = 6, N50% = 6, Ncontrol = 6; at 12 hr: N5% = 5, N10% = 6, N25% = 5, N50% = 5, Ncontrol = 5; at 24 hr: N5% = 7, N10% = 6, N25% = 7, N50% = 7, Ncontrol = 6; and at 48 hr: N5% = 7, N10% = 5, N25% = 7, N50% = 5, Ncontrol = 8.
High doses of ethanol increase mortality
For each ethanol treatment used in this study, we surveyed mortality at 0.5, 6, 12, 24, and 48 h post-ethanol consumption (Figure 5). We observed that mortality increased as a function of ethanol dose (Pois. reg. χ42 = 11.9, P = 0.018); however, in the post-hoc comparisons, only the 50% ethanol dose was significantly different from the control (P = 0.02).
Figure 5.
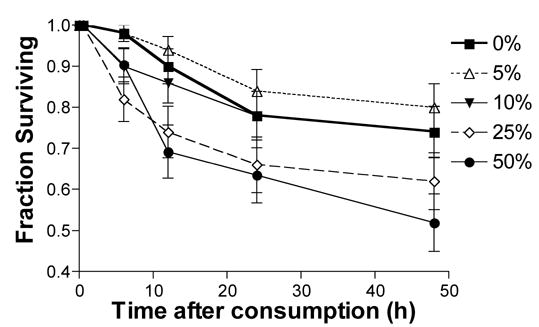
Mortality of honey bees consuming ethanol. The number of bees that died increased as ethanol dose increased (P = 0.018), however, MC showed that only subjects given 50% ethanol showed a significant increase in mortality. The fraction of subjects surviving at 0.5, 6, 12, 24 and 48 h post- ingestion is shown. Each point represents the mean +/− SEM of 50 honey bees. No deaths were observed at the 0.5 h time point.
Discussion
Using a detailed assay of honey bee behavior, we show that acute ethanol ingestion affects the locomotion and grooming activity in individual subjects in a time and dose-dependent manner. Ethanol-induced changes in behavior were characterized in general by less walking and by increases in stopped behavior and the amount of time spent upside down. Flying and grooming were also affected, though the relationship between ethanol dose and time spent engaged in these behaviors was more complex. Both the onset and the recovery experiments showed that subjects given higher doses of ethanol became inebriated more quickly and stayed inebriated longer. Furthermore, we demonstrated that recovery from ethanol inebriation occurred approximately 24–48 h post-ingestion, depending on the dose of ethanol received. Moreover, hemolymph ethanol levels correlated positively with ethanol dose, such that honey bees given the highest ethanol doses also had the greatest amount of ethanol in the hemolymph.
Ethanol effects on behavior are dose and time dependent
Previous investigations of ethanol’s molecular targets using Drosophila have measured both dose and time-dependent effects of ethanol on locomotion (Bainton, et al., 2000; Moore, et al., 1998; Parr, et al., 2001; Singh and Heberlein, 2000; Wolf, et al., 2002; see Guarnieri and Heberlein, 2003 for a review). Initial studies examining the effects of ethanol on the number of crossings in a shuttle-box and the turning of a “running wheel” suggested that ethanol consumption would also affect locomotion in honey bees (Abramson et al., 2000). Our studies are consistent with these results as honey bees given higher doses decreased the amount of time spent walking and increased time stopped. On the other hand, in humans, mice and Drosophila, low doses of ethanol lead to hyperactivity. In mice injected with a single dose of ethanol, walking speed is biphasic showing an initial increased followed by a decrease; the initial increase was associated with an increase in the duration of bouts of walking activity (Smoothy and Berry, 1984, 1985). Likewise, Wolf et al., (2002) determined that in Drosophila, hyperactivity was due to an increase in the time spent walking quickly and an increase in walking bout duration but not the frequency of bouts. Our assay examined the bout duration, frequency of the behavior, and the amount of time spent engaged in each behavior rather than directly measuring walking speed. Furthermore, control honey bees spent the vast majority of their time walking, making hyperactivity difficult to identify. However, as with mice and Drosophila, honey bees given 5% ethanol showed significant increases in walking bout duration during the first 40 min after consumption when compared to control bees, suggesting that they were exhibiting hyperactive behavior. In contrast, subjects given higher doses spent less time walking and showed significant decreases in both bout duration and frequency. Changes in walking behavior continued to be observed after the initial 40 min period, and by 6 h after ingestion, bees given any of the ethanol treatments showed a significant reduction in time spent walking.
Continuous observation of a single subject allowed us to characterize how ethanol changed both the duration and the pattern of specific behaviors after ingestion. Not only was locomotion (walking/stopped) behavior was affected by ethanol dose; we also found that ethanol also affected both the amount of time our subjects spent upside down and engaged in grooming behavior. In fact, time spent upside down was the strongest indicator of ethanol’s influence on honey bees. In Drosophila, flies given high doses of ethanol lie on their backs or sides and lose “postural control” such that they remain immobile and do not respond to mechanical stimulation (Rothenfluh and Heberlein, 2002; Wolf, et al., 2002). In contrast, the upside down behavior of honey bees occurred most often when a subject would fall from the top or side of the observation arena onto its back and was unable to restore itself to its feet. Upside down bees would lie on their backs, waving their legs in an attempt to right themselves, and were not comatose. Interestingly, we observed that the duration of bouts of time spent upside down was a function of both time and dose, but that the frequency of bouts of upside down behavior was not, suggesting that ethanol interferes with a honey bee’s ability to right itself rather than causing the subject to fall over more often.
One of the striking results from our study was that intermediate doses of ethanol affected grooming behavior in honey bees. The frequency and duration of bouts of grooming behavior for the intermediate doses (10–25%) in the first 40 min post-ingestion was greater than that of the control subjects, the low dose (5%) subjects and the higher dose (50–75%) subjects. At longer time points (4 – 6 h), bees given 25% ethanol reduced the amount of time spent grooming whereas 5% ethanol treated bees showed an increase in grooming behavior. The maximal point of inebriation occurred around 6 h post-ingestion (see section below), therefore, the dose-dependent shift in time spent grooming is consistent with high levels of grooming behavior in subjects receiving intermediate doses of ethanol. In contrast, acute ethanol administration did not affect bouts of self-grooming and ethanol suppressed bouts of abbreviated grooming in mice (Berry and Smoothy, 1985). The effects of ethanol on grooming have not been reported from previous studies of Drosophila or honey bees.
Ethanol’s role in disruption of central pattern generated behavior
The behaviors we assayed, walking (Delcomyn, 2004), flying (Wilson, 1961), grooming (Berkowitz and Laurent, 1996) and righting (upside down) (Sherman, et al., 1977; Zill, 1986), all proceed from rhythmic neural activity produced by central pattern generators (CPGs) in the central nervous system (for reviews see: Marder and Calabrese, 1996; Marder and Bucher, 2001; Marder, et al., 2005). That ethanol affects not only locomotion, but also grooming, righting and flying, strongly suggests that ethanol disrupts the central pattern generators underlying rhythmic behavior. Ethanol may affect central pattern generators via interference with neuromodulators, such as GABA, biogenic amines or neuropeptides, as each of these substances is involved in controlling the timing and feedback of these neural circuits (Alford, et al., 2003; Marder and Bucher, 2001). Indeed, previous studies have shown that one of ethanol’s primary effects on locomotion occurs via its interaction on the pathways involving these same neuromodulators (for reviews see Phillips and Shen 1996; Wolf and Heberlein, 2002). For example, studies using GABAA receptor knock out mice (see Boehm, et al., 2004 for a review) or Drosophila with reduced levels of GABAB receptors (Dzitoyeva, et al., 2003) suggest that ethanol affects locomotion and posture via modulation of GABAergic signaling. In addition, tyramine which influences Drosophila locomotion via CPGs (Fox, et al., 2006) also plays a role in ethanol induced hyperactivity (Scholz, 2005). Furthermore, consumption of ethanol leads to dopamine release in vertebrates (for reviews see: Phillips and Shen 1996; Tupala and Tiihonen, 2004) and dopamine has been shown to modulate a number of CPGs in both vertebrates and invertebrates (Kabotyanski, et al., 2000; Kiehn and Kjaerulff, 1996; Kloppenburg, et al., 1999).
Duration of the effects of acute ethanol consumption
Given the larger size of honey bees, we were able to directly measure the amount of ethanol in the hemolymph rather than determining ethanol levels for the entire animal as in studies with Drosophila or C. elegans. Hemolymph ethanol concentrations measured for the ethanol doses given to honey bees in our experiments were in the range of 40 to 100 mM, and are similar to the internal ethanol concentrations affecting locomotion in Drosophila (peaking around 50 to 60 mM; Moore, et al. 1998; Singh and Herberlein, 2000) and C. elegans (peak dose of approximately 30 mM; Davies, et al., 2003). However, the duration of inebriated behavior is longer than that observed for Drosophila or mice given an acute dose of ethanol. When Drosophila are exposed to ethanol as a vapor, recovery from ethanol-induced changes in behavior occurs within 5 h after ethanol vapor removal (Moore, et al., 1998; Singh and Herberlein, 2000). Mice injected with an acute ethanol dose recover behavior and return to baseline levels of blood ethanol approximately 3 h after administration (Smoothy and Berry, 1985). In contrast, honey bees given the high ethanol doses took 48 h to recover behavioral activities equal to the control subjects. We also observed that ethanol levels in the hemolymph of individual subjects remained at the maximum level up to 6 h after consumption and, for high doses, did not return to basal levels until between 24 and 48 h post-ingestion. The honey bees in our study had to be fed between the 24 and 48 h time points to prevent death, presumably due to starvation. It is possible that consumption of food influenced the recovery of bees given higher doses (25 or 50%) of ethanol. However, subjects treated with lower doses had already recovered by 24 h; therefore the recovery of these bees was not affected by the necessary feeding.
One possible reason for the persistence of inebriated behavior in honey bees compared to Drosophila or mice is that foraging honey bees use their crop to store solutions, such as nectar or water, for transportation back to the colony (Seeley, 1995). To maximize food transportation, honey bees carefully control the passage of material from storage in the impermeable crop into the midgut for digestion (Blatt and Roces, 2002; Crailsheim, 1988; Roces and Blatt, 1999; Visscher, et al., 1996). Thus, it is possible in our experiments that the absorption of the ethanol/sucrose solution was regulated such that ethanol passed into the midgut at a rate determined by the subject’s metabolic demands for sugar (Roces and Blatt, 1999; Blatt and Roces, 2002). Reducing the rate of crop emptying would prevent the immediate absorption of the entire ethanol dose and extend the period of inebriation. Another possible reason for the relatively long recovery time of our subjects is that honey bees may metabolize ethanol more slowly than Drosophila. In both mammals and insects, the main pathway for the metabolism of ethanol is via its oxidation to acetaldehyde by alcohol dehydrogenase (ADH), which is then converted to acetate by aldehyde dehydrogenase (ALDH). Expression levels and catalytic efficiency of ADH and ALDH show substantial variation within fruit fly populations suggesting that the genes responsible for producing these enzymes are subject to strong selective pressure (Fry, et al., 2004; Mercot, et al., 1994). Although honey bees possess ADH and ALDH enzymes (Martins, et al., 1977; Santos, et al., 2005), the expression levels and relative activity of these enzymes is unknown.
Conclusions
Ethanol has significant effects on motor function in mammals, and recent work in Drosophila and C.elegans has revealed that ethanol’s influence on locomotion extends to invertebrates as well. The work presented here serves to characterize time and dose dependent effects of ethanol on the locomotor behavior of the honey bee providing a basis for future work investigating the effects of ethanol on social behavior and learning and memory. Our work suggests that ethanol may also perturb the regulation of central pattern generated behaviors, although the mechanisms of its action remain to be investigated. The concentrations of ethanol used in this study induced a range of changes in behavior including severe defects in motor function; however, only honey bees given the highest doses showed a significant increase in mortality. This suggests that the range of ethanol concentrations used in this study did not produce irreversible damage, and that this range is appropriate for future studies focusing on the effects of ethanol on the honey bee nervous system.
Acknowledgments
The authors wish to thank Sue Cobey for maintaining the honey bee colonies, Shanza Mujeeb for help with the hemolymph ethanol assays, and Brian Smith for support and access to facilities. This work was supported by NIH (NIDA) grant DA017694 to JAM; GAW was supported in part by NSF agreement no. 0112050 awarded to the Mathematical Biosciences Institute (OSU) and in part by NIH (NCRR) grant awarded to B.H. Smith (9 R01 RR1466); and the research was also supported by Ohio State University College of Biological Sciences Dean’s Undergraduate Research Awards to ISM.
References
- Abramson CI, Stone SM, Ortez RA, Luccardi A, Vann KL, Hanig KD, Rice J. The development of an ethanol model using social insects I: behavior studies of the honey bee (Apis mellifera L.) Alcoholism: Clinical and Experimental Research. 2000;24:1153–1166. [PubMed] [Google Scholar]
- Alford S, Schwartz E, Viana di Prisco G. The pharmacology of vertebrate spinal central pattern generators. Neuroscientist. 2003;9:217–228. doi: 10.1177/1073858403009003014. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Current Biology. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Berkowitz A, Laurent G. Central generation of grooming motor patterns and interlimb coordination in locusts. Journal of Neuroscience. 1996;16:8079–8091. doi: 10.1523/JNEUROSCI.16-24-08079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt J, Roces F. The control of the proventriculus in the honeybee (Apis mellifera carnica L.) I. A dynamic process influenced by food quality and quantity? Journal of Insect Physiology. 2002;48:643–654. doi: 10.1016/s0022-1910(02)00090-2. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Ponomarev I, Jennings AW, Whiting PJ, Rosahl TW, Garrett EM, Blednov YA, Harris RA. gamma-Aminobutyric acid A receptor subunit mutant mice: new perspectives on alcohol actions. Biochemical Pharmacology. 2004;68:1581–1602. doi: 10.1016/j.bcp.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Crailsheim K. Regulation of food passage in the intestine of the honeybee (Apis mellifera L) Journal of Insect Physiology. 1988;34:85–90. [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- Delcomyn F. Insect walking and robotics. Annual Review of Entomology. 2004;49:51–70. doi: 10.1146/annurev.ento.49.061802.123257. [DOI] [PubMed] [Google Scholar]
- Dzitoyeva S, Dimitrijevic N, Manev H. Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence. Proceedings of the National Academy of Sciences USA. 2003;100:5485–5490. doi: 10.1073/pnas.0830111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox LE, Soll DR, Wu CF. Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine beta hydroxlyase mutation. Journal of Neuroscience. 2006;26:1486–1498. doi: 10.1523/JNEUROSCI.4749-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry JD, Bahnck CM, Mikucki M, Phadnis N, Slattery WC. Dietary ethanol mediates selection on aldeyde dehydrogenase activity in Drosophila melanogaster. Integrative and Comparative Biology. 2004;44:275–283. doi: 10.1093/icb/44.4.275. [DOI] [PubMed] [Google Scholar]
- Giurfa M. Cognitive neuroethology: dissecting non-elemental learning in a honeybee brain. Current Opinion in Neurobiology. 2003;13:726–735. doi: 10.1016/j.conb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Guarnieri DJ, Heberlein U. Drosophila melanogaster, a genetic model system for alcohol research. International Review of Neurobiology. 2003;54:199–228. doi: 10.1016/s0074-7742(03)54006-5. [DOI] [PubMed] [Google Scholar]
- Kabotyanski EA, Baxter DA, Cushman SJ, Byrne JH. Modulation of fictive feeding by dopamine and serotonin in aplysia. Journal of Neurophysiology. 2000;83:374–392. doi: 10.1152/jn.2000.83.1.374. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. Journal of Neurophysiology. 1996;75:1472–1482. doi: 10.1152/jn.1996.75.4.1472. [DOI] [PubMed] [Google Scholar]
- Kloppenburg P, Levini RM, Harris-Warrick RM. Dopamine modulates two potassium currents and inhibits the intrinsic firing properties of an identified motor neuron in a central pattern generator network. Journal of Neurophysiology. 1999;81:29–38. doi: 10.1152/jn.1999.81.1.29. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiological Reviews. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Current Biology. 2001;11:R986–996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Current Biology. 2005;15:R685–699. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Martins E, Mestriner MA, Contel EP. Alcohol dehydrogenase polymorphism in Apis mellifera. Biochemical Genetics. 1977;15:357–366. doi: 10.1007/BF00484466. [DOI] [PubMed] [Google Scholar]
- Menzel R. Searching for the memory trace in a mini-brain, the honeybee. Learning & Memory. 2001;8:53–62. doi: 10.1101/lm.38801. [DOI] [PubMed] [Google Scholar]
- Mercot H, Defaye D, Capy P, Pla E, David JR. Alcohol tolerance, ADH activity, and ecological niche of Drosophila species. Evolution. 1994;48:746–757. doi: 10.1111/j.1558-5646.1994.tb01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Morgan PG, Sedensky MM. Mutations affecting sensitivity to ethanol in the nematode, Caenorhabditis elegans. Alcoholism: Clinical and Experimental Research. 1995;19:1423–1429. doi: 10.1111/j.1530-0277.1995.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Parr J, Large A, Wang X, Fowler SC, Ratzlaff KL, Ruden DM. The inebriactometer: a device for measuring the locomotor activity of Drosophila exposed to ethanol vapor. Journal of Neuroscience Methods. 2001;107:93–99. doi: 10.1016/s0165-0270(01)00357-0. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Shen EH. Neurochemical bases of locomotion and ethanol stimulant effects. International Review of Neurobiology. 1996;39:243–282. doi: 10.1016/s0074-7742(08)60669-8. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Grozinger CM, Whitfield CW. Sociogenomics: social life in molecular terms. Nature Reviews Genetics. 2005;6:257–270. doi: 10.1038/nrg1575. [DOI] [PubMed] [Google Scholar]
- Roces F, Blatt J. Haemolymph sugars and the control of the proventriculus in the honey bee Apis mellifera. Journal of Insect Physiology. 1999;45:221–229. doi: 10.1016/s0022-1910(98)00116-4. [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Heberlein U. Drugs, flies, and videotape: the effects of ethanol and cocaine on Drosophila locomotion. Current Opinion in Neurobiology. 2002;12:639–645. doi: 10.1016/s0959-4388(02)00380-x. [DOI] [PubMed] [Google Scholar]
- Santos KS, dos Santos LD, Mendes MA, de Souza BM, Malaspina O, Palma MS. Profiling the proteome complement of the secretion from hypopharyngeal gland of Africanized nurse-honeybees (Apis mellifera L.) Insect Biochemistry and Molecular Biology. 2005;35:85–91. doi: 10.1016/j.ibmb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Scholz H. Influence of the biogenic amine tyramine on ethanol-induced behaviors in Drosophila. Journal of Neurobiology. 2005;63:199–214. doi: 10.1002/neu.20127. [DOI] [PubMed] [Google Scholar]
- Scholz H, Franz M, Heberlein U. The hangover gene defines a stress pathway required for ethanol tolerance development. Nature. 2005;436:845–847. doi: 10.1038/nature03864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley TD. Honeybee ecology. Princeton University Press; Princeton: 1985. [Google Scholar]
- Seeley TD. The wisdom of the hive. Harvard University Press; Cambridge: 1995. [Google Scholar]
- Sherman E, Novotny M, Camhi JM. A modified walking rhythm employed during righting behavior in the cockroach Gromphadorhina portentosa. Journal of Comparative Physiology. 1977;113:303–316. [Google Scholar]
- Singh CM, Heberlein U. Genetic control of acute ethanol-induced behaviors in Drosophila. Alcoholism: Clinical and Experimental Research. 2000;24:1127–1136. [PubMed] [Google Scholar]
- Smoothy R, Berry MS. Alcohol increases both locomotion and immobility in mice: an ethological analysis of spontaneous motor activity. Psychopharmacology (Berl) 1984;83:272–276. doi: 10.1007/BF00464793. [DOI] [PubMed] [Google Scholar]
- Smoothy R, Berry MS. Time course of the locomotor stimulant and depressant effects of a single low dose of ethanol in mice. Psychopharmacology (Berl) 1985;85:57–61. doi: 10.1007/BF00427322. [DOI] [PubMed] [Google Scholar]
- Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Progress in Neuropsychopharmacology & Biological Psychiatry. 2004;28:1221–1247. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Visscher PK, Crailsheim K, Sherman G. How do honey bees (Apis mellifera) fuel their water foraging flights. Journal of Insect Physiology. 1996;42:1089–1094. [Google Scholar]
- Wilson DM. The central nervous control of flight in a locust. Journal of Experimental Biology. 1961;38:471–490. [Google Scholar]
- Winston ML. The biology of the honey bee. Harvard University Press; Cambridge: 1987. [Google Scholar]
- Wolf FW, Rodan AR, Tsai LT, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. Journal of Neuroscience. 2002;22:11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf FW, Heberlein U. Invertebrate models of drug abuse. Journal of Neurobiology. 2003;54:161–178. doi: 10.1002/neu.10166. [DOI] [PubMed] [Google Scholar]
- Zill SN. A model of pattern generation of cockroach walking reconsidered. Journal of Neurobiology. 1986;17:317–328. doi: 10.1002/neu.480170406. [DOI] [PubMed] [Google Scholar]


