Abstract
Oleamide (cis-9-octadecenamide) is a member of an emerging class of lipid-signaling molecules, the primary fatty acid amides. A growing body of evidence indicates that oleamide mediates fundamental neurochemical processes including sleep, thermoregulation, and nociception. Nevertheless, the mechanism for oleamide biosynthesis remains unknown. The leading hypothesis holds that oleamide is synthesized from oleoylglycine via the actions of the peptide amidating enzyme, peptidylglycine alpha amidating monooxygenase (PAM). The present study investigated this hypothesis using pharmacologic treatments, physiologic assessments, and measurements of serum oleamide levels using a newly development enzyme-linked immunosorbant assay (ELISA). Oleamide and oleoylglycine both induced profound hypothermia and decreased locomotion, over equivalent dose ranges and time courses, whereas, closely related compounds, stearamide and oleic acid, were essentially without effect. While the biologic actions of oleamide and oleoylglycine were equivalent, the two compounds differed dramatically with respect to their effects on serum levels of oleamide. Oleamide administration (80 mg/kg) elevated blood-borne oleamide by eight-fold, whereas, the same dose of oleoylglycine had no effect on circulating oleamide levels. In addition, pretreatment with the established PAM inhibitor, disulfiram, produced modest reductions in the hypothermic responses to both oleoylglycine and oleamide, suggesting that the effects of disulfiram were not mediated through inhibition of PAM and a resulting decrease in the formation of oleamide from oleoylglycine. Collectively, these findings raise the possibilities that: (1) oleoylglycine possesses biologic activity that is independent of its conversion to oleamide, and (2) the increased availability of oleoylglycine as a potential substrate does not drive the biosynthesis of oleamide.
INTRODUCTION
Cis-9-octadecenamide, commonly referred to as oleamide, is the prototype member of an emerging class of lipid messengers, the primary fatty acid amides [1]. A growing body of evidence indicates that oleamide mediates fundamental neurochemical processes [2-4] with resulting physiologic responses that include sleep [5-8], thermoregulation [9-11], and nociception [9]. Oleamide was first identified in 1989 as one of six primary fatty acid amides present in normal human serum [12]. Subsequently, oleamide was shown to accumulate in the cerebrospinal fluid of sleep-deprived cats and to produce a profound sleep-like state when administered to rodents [5,6,13]. Based upon these findings it was proposed that oleamide contributes to the biochemical mechanisms underlying the drive for sleep [5,6,13].
Despite the potential importance of oleamide in the genesis of sleep and other fundamental behaviors, the mechanism for its biosynthesis remains to be defined. At present, two hypotheses are driving research on the biosynthetic pathway for oleamide. The leading hypothesis holds that that the neuropeptide processing enzyme, peptidylglycine alpha-amidating monooxygenase (PAM) is the mediator of oleamide biosynthesis. Merkler and colleagues [14] were the first to show that PAM does, in fact, catalyzes the formation of oleamide from Noleoylglycine in vitro. A less likely second hypothesis proposes that fatty acid amide hydrolase (FAAH), the enzyme recognized for its role in the catabolic regulation of oleamide, mediates the synthesis of oleamide. While FAAH appears capable of working in reverse to produce oleamide from oleic acid and ammonia in vitro [15,16], the high concentrations of ammonia required make this an improbable physiologic mechanism.
PAM is a bifunctional enzyme that is widely expressed in neuroendocrine tissues where it catalyzes formation of alpha-amidated peptide messengers from their glycine-extended precursors [17,18]. Beyond an absolute requirement for a reactive glycine residue that serves as the amide donor [17], PAM exhibits little substrate specificity. Each naturally occurring amino acid is found as a carboxy terminal amide in peptide messengers of the neural and endocrine systems [17]. Additionally, PAM is capable of catalyzing the amidation of a variety of non-peptide compounds that contain a reactive glycine [17]. Included in this group of potential biologic substrates are glutathione [19], leucotriene C4 [19], and bile acids [20,21]. While amidation of these compounds is readily demonstrated experimentally, PAM's role in generating novel bioactive amides in vivo has yet to be established. Likewise, the role of PAM in the in vivo generation of oleamide remains in question. Extensive efforts of this and other laboratories have failed to identify the key enzyme for generating N-oleoylglycine. Nevertheless, the production of N-oleoylglycine by a recombinant bile acid conjugating enzyme [22] and by cultured neuroblastoma cells [23] indicate the feasibility for a PAM-based mechanism for the generation of oleamide in vivo.
An important limitation to our understanding of oleamide biosynthesis is the absence of a sensitive, reliable, specific, and quantitative assay for its measurement. The approaches currently available rely upon complex gas chromatographic-mass spectral procedures [16,23,24] which are cumbersome and expensive. To address these limitations we have developed a highly specific enzyme-linked immunosorbant assay (ELISA) for measuring blood levels of oleamide. Using this assay, we report here that while N-oleoylglycine was equipotent with oleamide in decreasing locomotion and hypothermia, it failed to increase circulating levels of oleamide. In contrast, an equivalent dose of oleamide increased circulating concentrations of oleamide 8-fold. Accordingly, the findings raise the possibilities that: (1) oleoylglycine is bioactive independent of conversion to oleamide, and (2) availability of oleoylglycine as a substrate does not limit the in vivo biosynthesis of oleamide.
MATERIALS AND METHODS
Chemicals and Special Supplies
Acetone, ammonium hydroxide, keyhole limpet hemocyanin (KLH), bovine serum albumin (BSA), human transferrin, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), dimethyl formamide (DMF), Sigma Fast™ p-nitrophenyl phosphate (PNPP) tablet set, sodium azide, sodium borohydride (NaBH4), sodium carbonate (Na2CO3), sodium bicarbonate (NaHCO3), ammonium sulfate ([NH4]2SO3), 2-[N-morphoplino]ethanesulfonic acid (MES), tris[hydroxymethyl]aminomethane (Tris), polyoxyethylenesorbitan monolaurate (Tween®20), polyoxyethylenesorbitan monooleate (Tween®80), and tetraethylthiuram disulfide (disulfiram) were obtained from Sigma Chemical Co. (St. Louis, MO). Erucamide (13-docosenamide), oleic acid, oleamide (9-octadecenamide), oleamide-18-aldehyde (ODA-18-Ald, as a custom synthesis), N-oleoylglycine, stearamide, and mouse anti-rabbit IgG precoated microtiter plates were obtained from Cayman Chemical (Ann Arbor, MI). Dimethyl formamide (DMF), EZ-Link™ biocytin hydrazide, goat anti-rabbit alkaline phosphatase, NeutrAvidin™ conjugated to alkaline phophatase and SuperBlock® were acquired from Pierce (Rockford, IL). Strata™ C18-E columns were obtained from Phenomenex (Torrance, CA). [oleic acid-1-14C]Oleamide (50.5 mCi/mmol) and [oleic acid-1-14C]-N-oleoylglycine (51 mCi/mmol) were prepared as custom syntheses by NEN Life Science Products (Boston, MA). [1-14C]Oleic acid, anti-rabbit IgG conjugated to horseradish peroxidase and ECL Plus Western Blotting Detection Reagents were purchased from Amersham Biosciences (Piscataway, NJ). All reagents were the highest quality commercially available.
In Vivo Experimental Designs, Data Collection and Analysis
Animals and treatments
Male Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing 250-300 g were housed under a 12-h daily reverse light cycle and received food and water ad libitum. Oleamide, stearamide and oleic acid were dissolved in peanut oil; oleoylglycine was dissolved a 1:1 mixture of peanut oil and 0.9% NaCl adjusted to pH 7.0 with 0.1 N sodium hydroxide. Solutions were heated to 70°C to thoroughly dissolve the lipids, cooled to 40°C and administered as single intraperitoneal injections (1 mL). Control animals received injections of peanut oil vehicle only. Disulfiram was prepared in 0.9% saline containing 0.5% Tween 80 and administered daily for 7 days by subcutaneous injection (400 mg/kg). Control animals received injections of vehicle only. Blood was collected into siliconized tubes from animals anesthetized with CO2 gas and decapitated by guillotine.
Body temperature measurement
Core body temperatures were measured using a YSI Model 47 Tele-Thermometer (Yellow Springs Instrument Co., Yellow Springs, OH) equipped with a colonic thermal probe (2.5 cm).
Motor activity measurement
Locomotor activity was measured during the dark phase (2 h after lights out) using an Omnitech Electronics Digiscan infrared photocell system (Test box model RXYZCM [16 TAO]; Omnitech Electronics, Columbus, OH), located in a dedicated room. Animals were placed singly in a 40×40×30 cm clear Plexiglas arena with a ventilated lid. A photocell array measured horizontal locomotor activity using 16 pairs of infrared photocells located every 2.5 cm from side-to-side and 16 pairs of infrared photocells located front-to-back in a plane 2 cm above the floor of the arena. Data were automatically gathered and transmitted to a computer via an Omnitech Model DCM-I-BBU analyzer. The apparatus monitored animal activity continuously with data recorded as cumulative activity every 5 min for a total testing period of 1 h. The testing room remained dark and the subjects were undisturbed during the testing period. Total activity for control animals was 22,195 +/- 2563 counts over the sixty minute recording period.
Statistical Analyses
Data were analyzed using repeated-measures analyses of variance (ANOVA) and Dunnett's two-tailed post-hoc testing. Significance was set at p<0.05.
Development of Anti-Oleamide Antisera
Preparation of oleamide-protein conjugates for immunization and antisera characterization
KLH, transferrin, and BSA were prepared as stock solutions in 20 mM Na2CO3 at 10 mg/mL. ODA-18-Ald was prepared as a stock solution in DMF at 40 mg/mL. Stock protein solutions (0.5 mL) were diluted four-fold to 2.5 mg/mL with DMF (75% final, 2 mL). To each solution 60 μL, 70 μL or 80 μL of stock ODA-18-Ald was added for KLH, transferrin and BSA, respectively. These amounts represent 2- to 3-fold molar excess of aldehyde over the protein lysine content. The reaction mixture was incubated at 37°C overnight. Control reactions were performed in parallel using unmodified oleamide in place of ODA-18-Ald. The reactions were reduced by the addition 50 μL of 20 mg/mL NaBH4 in 20 mM Na2CO3 and continued incubation at 37°C for an additional 1-2 h. Residual NaBH4 was quenched by the addition of 10 μL of acetone and incubation for 30 min at room temperature. The reaction mixtures were then dialyzed (SnakeSkin™ dialysis tubing, 10,000 mw cut off, Pierce) against 5 liters of 20 mM Na2CO3 for 16 h at room temperature. The dialysates were collected, and the precipitates that formed during dialysis were removed by centrifugation. Protein concentration was determined by the method of Lowry et al., [26]. Conjugates were stored at 4°C to avoid precipitation that occurred with freezing.
Preparation of colloidal gold-oleamide-protein complex and immunization
Anti-oleamide antisera were developed in rabbits and guinea pigs utilizing three separate commercial services (Cytimmune Sciences, Inc., Rockville, MD, Covance, Inc., Denver, PA and Cayman Chemical). The highest titer and most sensitive antisera, ODA-1, was developed by Cytimmune Sciences, Inc., as follows. Oleamide-protein conjugate was diluted to 200 μg/ml protein with water and then combined with an equal volume of 10 nanometer colloidal gold, 10 ppm and reacted by gentle mixing for 10 minutes at room temperature. The protein/gold complex was then emulsified in an equal volume of Fruend's complete adjuvant by passage through a narrow bore stop cock between two syringes. The resulting emulsion was administered at multiple subcutaneous sites (15 - 20) to adult New Zealand white rabbits (1.0 mL/rabbit). Booster immunizations were similarly prepared and administered in Freund's incomplete adjuvant at 6 to 8 week intervals. Antisera were collected periodically by ear vein venipuncture. Maximal antibody titers were obtained by four months of immunization.
Screening anti-oleamide antibody by dot blot
Proteins and protein-oleamide conjugates (3-5 μg in 5 μL) were spotted to Nytran® membrane (Schleicher & Schuell, Keene, NH) and air dried. The membrane was blocked for 1 h at room temperature with 1% nonfat dried milk in Tris-Buffered Saline-Tween®20 (TBS-T, 50 mM Tris, pH 7.6, 0.8% NaCl, 0.1% Tween®20) (blocking buffer) and then incubated with anti-oleamide antibody (1:4000 dilution) overnight at 4°C. Following extensive washing with TBS-T, the blot was incubated with anti-rabbit IgG-horseradish peroxidase conjugate (diluted 1:10,000 in blocking buffer) for 2 h at room temperature. After thorough washing with TBS-T, the blot was reacted with ECL Plus reagents according to the manufacturer's instructions, and immunoreactive spots were visualized by exposure to photographic film.
Titering of antisera
Antisera identified by dot blot were further evaluated for their ability to bind oleamide in an ELISA-based system. Oleamide-BSA conjugate (1 μg/mL) in plate coating buffer (150 mM Na2CO3, 350 mM NaHCO3, pH 9.6), was immobilize in wells (100 μL/well) of a plastic microtiter plates (Nunc MaxiSorp™ 96-well, flat-bottom; Thomas Scientific, Swedesboro, NJ) by incubation overnight at room temperature. Plates were washed with TBS-T and blocked for 2 hours at room temperature with 300 μL/well of ELISA blocking buffer (SuperBlock® prepared according to the manufacturer's instructions and supplemented with 0.05% Tween-20 and 0.01% sodium azide). The plates were washed and then incubated with serial dilutions of the antisera in ELISA blocking buffer (100 μL/well) overnight at room temperature. After washing with TBS-T, 100 μL of goat anti-rabbit alkaline phosphatase conjugate (1 μg/mL) in ELISA blocking buffer was added to each well for 2 hours. The plate was washed and PNPP substrate solution (1 mg/mL PNPP in 200 mM Tris, pH 10.0) was added (100 μL/well). The color was allowed to develop over 10-30 min and absorption read at 405 nm using a Sunrise™ multi-channel, tuneable wavelength absorbance reader (Tecan, Research Triangle Park, NC). The titer of the antiserum was defined as the dilution of antiserum which produced 50% of maximal signal.
Purification of IgG fraction from rabbit antisera
The IgG fractions were purified from high titer antisera by ion exchange HPLC using a Wide-Pore ABx, 15 μ, 10 × 250 mm column (J.T. Baker, Phillipsburg, NJ). Sera were prepared for chromatography by dialysis against HPLC buffer A (25 mM MES, pH 5.6) for 4 h and cleared of precipitate by centrifugation. The samples were applied to the column equilibrated in buffer A and eluted with a step gradient of buffer B (1 M [NH4]2SO4 in 25 mM MES, pH 7.0) as follows: 0% B from 0 to 10 min, 5% B from 10 to 20 min, 25% from 20 to 30 min followed by a linear gradient to 100% B at 40 min. The IgG containing fractions were pooled and dialyzed against TBS overnight and then passed through a 22 um filter. Sodium azide was added to 0.1% as a preservative and the purified IgG was stored in aliquots at -20°C. To maintain the integrity of the purified antibody during repeated freezing and thawing, a working aliquot was prepared by mixing the IgG preparation with an equal volume of glycerol.
The Immunoassay for Oleamide
Overview of the assay
The oleamide ELISA is a competitive binding assay in which oleamide and biotinylated oleamide compete for binding to anti-oleamide IgG immobilized in wells of a microtiter plate. Detection is accomplished by avidin-alkaline phosphatase conjugate and subsequent reaction with PNPP substrate.
Preparation of biotin-oleamide conjugate
Biotin was conjugated to the ODA-18-Ald using 1.2 mg of biocytin hydrazide in 200 μL water. DMF was added to the biocytin solution in 6×100 μL aliquots with mixing in between to a final concentration of 75% DMF. ODA-18-Ald (40 mg/mL in DMF) was added to the biocytin hydrazide in a volume of 9.6 μL. This represents a 2:1 stoichiometric ratio of biocytin hydrazide to ODA-18-Ald. The reaction was allowed to proceed overnight at room temperature with constant agitation on a magnetic stirrer. The reaction volume was concentrated to a volume of 50 μL under a stream of nitrogen. Excess unreacted biocytin hydrazide was precipitated with the addition of 450 μL of ethanol and removed by centrifugation. Synthesis of biotin-oleamide was confirmed by electrospray mass spectrometry using a Finnigan TSQ triple stage quadrupole mass spectrometer (Thermo Electron Corp., Waltham, MA) operated in positive ion mode. For analysis the sample was acidified with acetic acid (final concentration 0.5%). Scans were made in the range of 10-1000 m/z and accumulated for 8 sec. The major peaks in the spectrum were oleamide-biotin (m/z = 664.6) and unreacted biocytin hydrazide (m/z = 387.35). There was no evidence of residual ODA-18-Ald (m/z = 296.5) in the mass spectrum. Therefore, assuming 100% biotinylation of the OAD-18-Ald, the final concentration of oleamide-biotin was 2.6 mM.
Optimization of oleamide ELISA components
The oleamide ELISA was optimized by determining the antibody and biotinylated oleamide concentrations necessary to achieve maximal signal and highest sensitivity. All incubations were conducted at room temperature. Serial dilutions of anti-oleamide IgG were made in ELISA blocking buffer and applied (100 μL per well) to a pre-blocked mouse anti-rabbit IgG coated microtiter plate. The plate was incubated for 3 h, washed with TBS-T and reacted overnight with an excess of oleamide-biotin in ELISA binding buffer (TBS, 0.05% CHAPS, 10% ethanol, 100 μL per well). The plate was then: washed; incubated with NeutrAvidin-alkaline phosphatase conjugate (1 μg/mL in ELISA blocking buffer, 100 μL per well, 2 h); washed; reacted with PNPP substrate (100 μL per well 20-40 min) and then read for absorbance at 405 nm. Based upon the degree of developed absorbance, a 1:40,000 dilution of ODA-1 IgG was selected for the ELISA. An optimal concentration of oleamide-biotin was then similarly determined to be 1:100,000 using plates prepared with the 1:40,000 working dilution of ODA-1 IgG.
Generation of standard curves and specificity testing
Stock oleamide standards from 5 mM to 0.01 mM were prepared as two-fold serial dilutions in 100% ethanol and stored at 4°C. Each standard was diluted 1:1,000 in ELISA binding buffer containing 1:100,000 dilution of biotin-oleamide. An assay plate was prepared by applying anti-oleamide IgG (1:40,000 dilution in ELISA blocking buffer, 100 μL per well) to a pre-blocked mouse anti-rabbit IgG coated plate and incubating for 3-5 h. After washing, 100 μL of the diluted oleamide standards were applied to replicate wells and incubated overnight. The plate was washed and incubated with NeutrAvidin-alkaline phosphatase conjugate (1 μg/mL in ELISA blocking buffer, 100 μL per well) for 2 h. Following a final wash, the plate was incubated with PNPP substrate solution (100 uL per well), and the absorbance at 405 nm of each well was measured. The standard curve was generated by plotting B/Bo (A405nm − NSB ÷ maximum A405nm − NSB) for each data point against the value of the oleamide standard concentration. For specificity analyses, compounds were similarly diluted in ethanol and incorporated into the assay exactly as described above. Standard curves used for estimating serum concentrations of oleamide were prepared in an identical manner to that used for assaying experimental serum samples. Specifically, equivalent volume aliquots from a pool of rat serum with an oleamide concentration below the limit of the assay (less than 10 ng/ml) were spiked with known amounts of oleamide standard prior to extraction and incorporation into the ELISA. In this manner, internal standard curves were generated for each experiment to precisely match the analysis conditions of the experimental serum samples.
Preparation of Serum samples for ELISA assay
Oleamide Extraction
A serum extraction procedure was necessary to isolate oleamide from other unknown substances that interfere in the ELISA assay. Siliconized glass tubes, vials and pipettes were used throughout. Serum (routinely 200 μL) was extracted by vortexing with two volumes of acetone and incubating at −20°C for 20 minutes. Precipitated protein was removed by centrifugation and supernatants collected into 12×75 mm glass tubes. Acetone was removed under a gentle stream of nitrogen and samples were then extracted with 1.5 mL of chloroform : methanol (2:1) by vortexing. Following centrifugation, the oleamide-containing lower phase was transferred to a new tube, dried under nitrogen, resuspended in 400 μL of 50% methanol and applied to a Strata™ C18-E column equilibrated in 50% methanol following preconditioning with a serum extract using a pool of control rat serum. Following sample application, the column was sequentially washed with 80% methanol (600 μL) and 80% acetonitrile (600 μL) and then eluted with of 100% ethyl acetate (500 μL). The ethyl acetate eluate was dried under nitrogen and redissolved in 100 μL of ELISA binding buffer containing 1:100,000 dilution of biotin-oleamide and incorporated into the ELISA. Serum oleamide values were determined by reference to an internal standard curve run in parallel with the experimental samples. Recovery of [14C]oleamide through the extraction was 45 ± 1% (N=17).
Thin Layer Chromatography
Samples in 100% ethanol were spotted onto the preadsorbent zone of channeled, Silica Gel G, 250 μ, 20×20 cm TLC plates (Analtech, Newark, DE) and developed for 1.5 h in chloroform : methanol : water (90:10:1) containing 20 μL/100 mL ammonium hydroxide (18). Radioactivity on the TLC plates was visualized by phosphorimaging using a Typhoon 9410 Variable Mode Imager (Amersham Pharmacia Biotech, Piscataway, NJ) and analyzed using ImageQuant version 5.2 software (Amersham).
RESULTS
Immunogenic conjugates of oleamide were produced by coupling ODA-18 aldehyde to KLH, BSA or transferrin as describe under Material and Methods. Antisera were developed in both rabbits and guinea pigs. The antisera possessing the highest specificity, affinity and titer, designated as ODA-1, was developed in a rabbit against oleamide-KLH-colloidal gold conjugate. The IgG fraction was purified from this antiserum and used for these studies. Figure 1 demonstrates the presence of anti-oleamide antibodies by western dot-blot analysis. Native BSA, KLH and transferrin (TF), and their respective oleamide conjugates, were spotted onto Nytran® membrane and reacted with ODA-1 antibody. Bound anti-oleamide antibody was detected by probing with anti-rabbit IgG horseradish peroxidase conjugate and visualization by enhanced chemiluminesence. As shown, ODA-1 antibodies reacted with all three oleamide containing protein conjugates, as well as native KLH, the protein carrier used to produce the immunogen. In contrast, ODA-1 failed to recognize native BSA or TF, thus demonstrating specific reactivity for oleamide.
Figure 1.
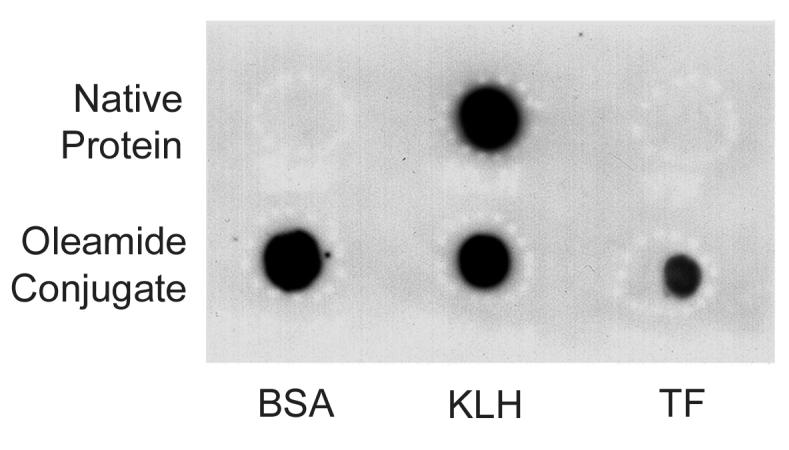
Dot blot screening for anti-oleamide antibodies. Equivalent amounts (3-5 μg) of native bovine serum albumin (BSA), keyhole limpet hemocyanin (KLH) and transferrin (TF) proteins (top row), or their respective oleamide conjugates (bottom row) were spotted to Nytran® membrane. The blot was blocked and then incubated with antibody raised against oleamide conjugated to KLH (ODA-1 diluted 1:4,000). Following extensive washing, bound primary antibody was visualized by enhanced chemiluminesence using a goat anti-rabbit secondary antibody coupled to horseradish peroxidase.
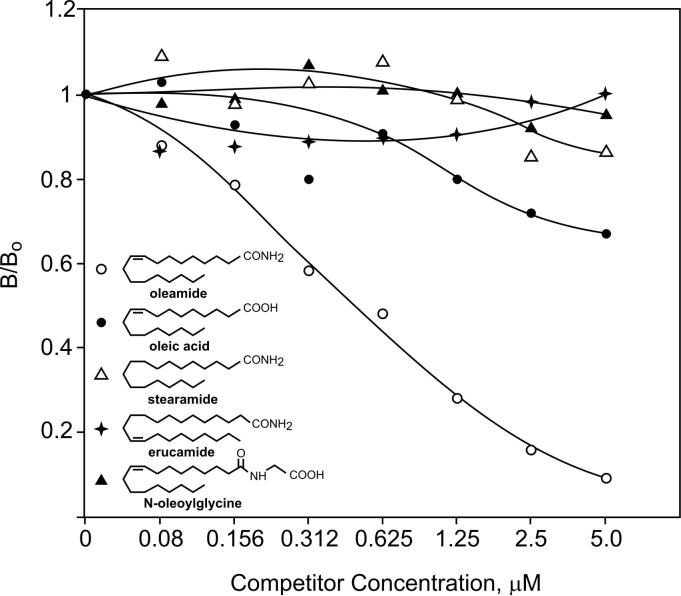
ODA-1 IgG was used to develop an enzyme-linked immunosorbant assay (ELISA) for oleamide as described under Materials and Methods. Using this competition assay, ODA-1 demonstrated remarkable specificity for oleamide (Fig. 2). When compared to four closely related compounds (structures shown in Fig. 2), only oleamide showed significant reactivity with the antibody. Oleic acid, however, did show slight affinity for the antibody at the highest concentrations tested. Specifically, these results demonstrate that the terminal amide and 9-10 double bond are required for recognition by ODA-1.
Figure 2.
Specificity of ODA-1 antibody for oleamide and structurally related compounds by ELISA. Oleamide (open circles), oleic acid (closed circles), stearamide (open triangles), erucamide (stars) and N-oleoylglycine (closed triangles) were used as competing ligands in the oleamide ELISA at the concentration indicated. ODA-1 IgG and oleamide-biotin tracer were used at dilutions of 1:40,000 and 1:100,000, respectively (see Materials and Methods for additional details).
Figure 3 demonstrates the ability of the ODA-1 ELISA to measure oleamide in serum. The concentration-dependent competition curve was generated from 200 uL aliquots of pooled rat serum to which known amounts of oleamide standard had been added. The samples were extracted and incorporated into the ELISA. In this manner, standard and experimental values were normalized for loss through the extraction, and the standard curve precisely matched the conditions of the experimental samples. It can be seen from the data in Figures 2 and 3 that the limit of sensitivity for the oleamide ELISA is at least 0.05 μM or 1.4 ng/100μL. The stability of oleamide in serum was verified by thin layer chromatography (TLC) and phosphorimaging. Figure 4 shows the fractionation of [14C]oleamide, [14C]N-oleoylglycine and [14C]oleic acid added to 1% rat serum and incubated at room temperature for either 0 min or 60 min. Serum extracts were prepared and subjected to TLC analysis as detailed under Material and Methods. Both oleamide and oleic acid were stable in rat serum, whereas, N-oleoylglycine underwent a significant degree of degradation (approximately 20%) to produce oleic acid. [14C]Oleamide was similarly stable in 100% rat serum stored at 4°C for three days (data not shown). Based upon these findings it was concluded that oleamide is stable in rat serum under the experimental conditions employed here.
Figure 3.
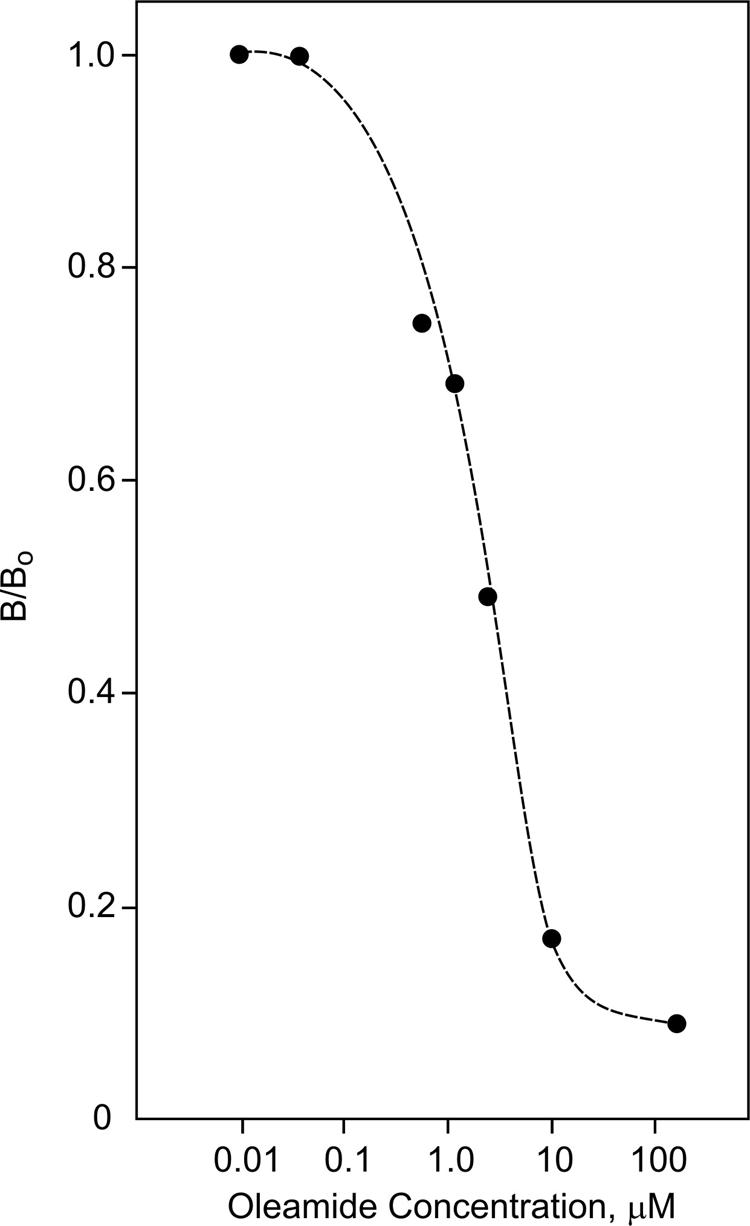
ELISA standard curve for oleamide extracted from serum. Aliquots of rat serum (200 uL) were supplemented with known amounts of oleamide (points plotted) and extracted as described under Materials and Methods. The extracts were used in the ODA-1 competition ELISA to generate the standard curve as shown. Data points are the average of duplicate determinations.
Figure 4.
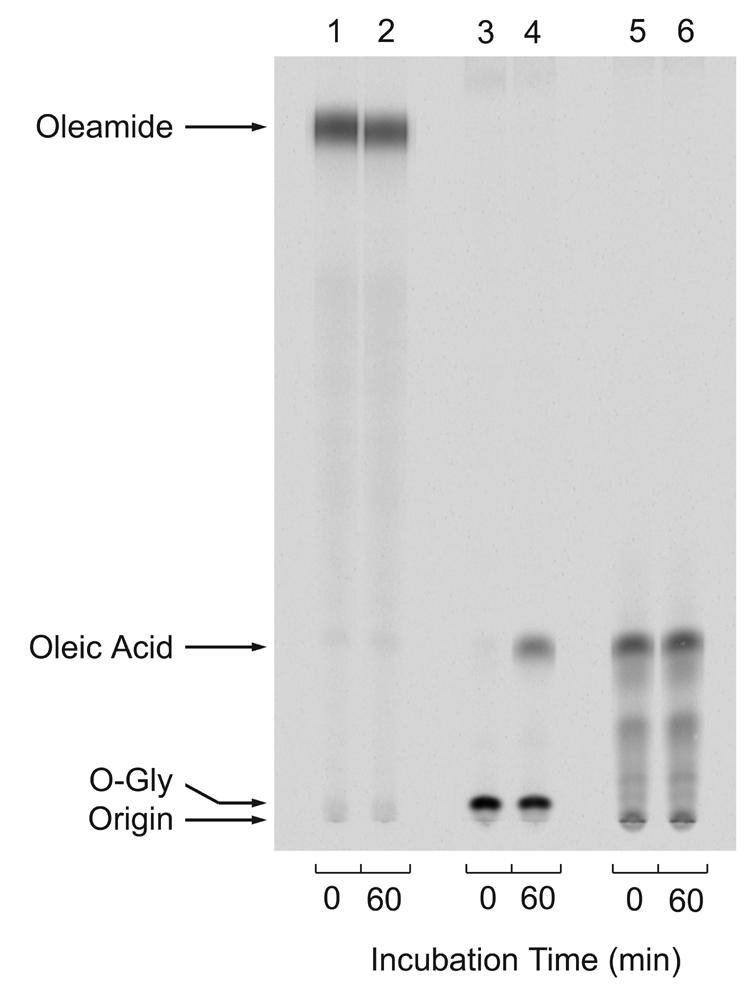
Stability of oleamide in rat serum. [14C]Oleamide (lanes 1 and 2), [14C]N-oleoylglycine (lanes 3 and 4) or [14C]oleic acid (lanes 5 and 6)(80,0000 cpm each) were incubated at room temperature in 50 μL TBS containing 0.5 μL of rat serum for either 0 min (lanes 1,3,5) or 60 min (lanes 2, 4, 6). Samples were first extracted with 5 parts ethyl acetate followed by a second extraction with 5 parts hexane/ethyl acetate 1:2. The organic phases were pooled and dried under a stream of nitrogen. Each sample was dissolved in 10 μL of ethanol and applied to a silica TLC plate. The plate was developed as described under Materials and Methods, and the radioactivity was visualized by phosphorimager.
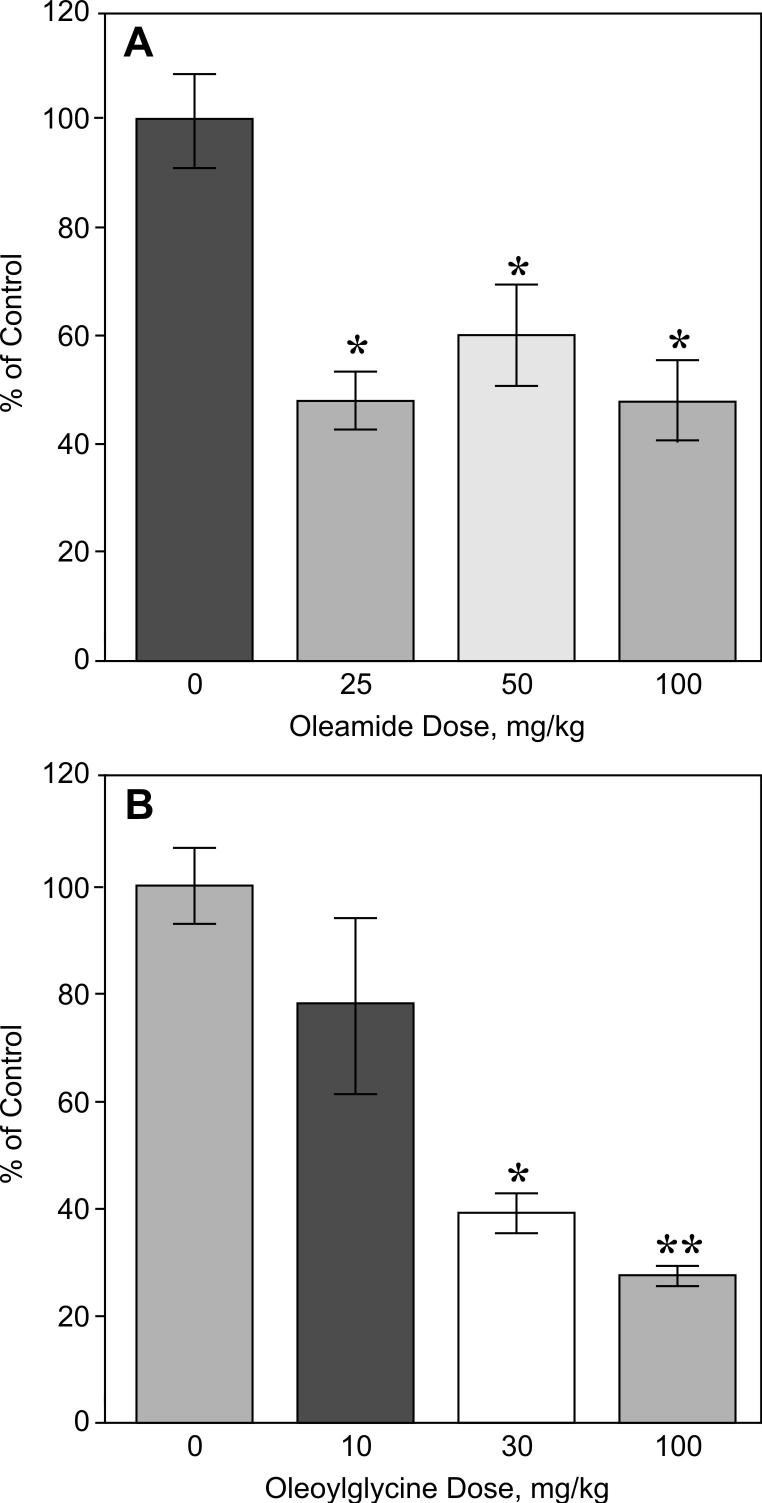
The oleamide ELISA was then used to test the hypothesis that oleamide is derived, in vivo, from N-oleoylglycine by the enzymatic action of PAM. Figure 5 presents the effects of Noleoylglycine and oleamide administration (80 mg/kg, intraperitoneal, 60 min) on serum levels of oleamide in rats. Circulating levels of oleamide increased eight-fold (209 ± 65 ng/mL) following the administration of exogenous oleamide compared to the vehicle injected control group (16 ± 4 ng/ml) (p < 0.01). In contrast, administration of N-oleoylglycine did not result in a significant change in blood levels of oleamide (27 ± 6 ng/mL). Despite the failure of administered N-oleoylglycine to increase circulating oleamide, the two agents were very similar with respect to their behavioral actions. Specifically, oleamide and N-oleoylglycine exhibited a similar threshold for reducing motor activity (Fig. 6). However, while the effect of oleamide on locomotion plateaued at intermediate doses (Fig. 6, panel A), increasing amounts of Noleoylglycine produced successive declines in motor activity (Fig. 6, panel B).
Figure 5.
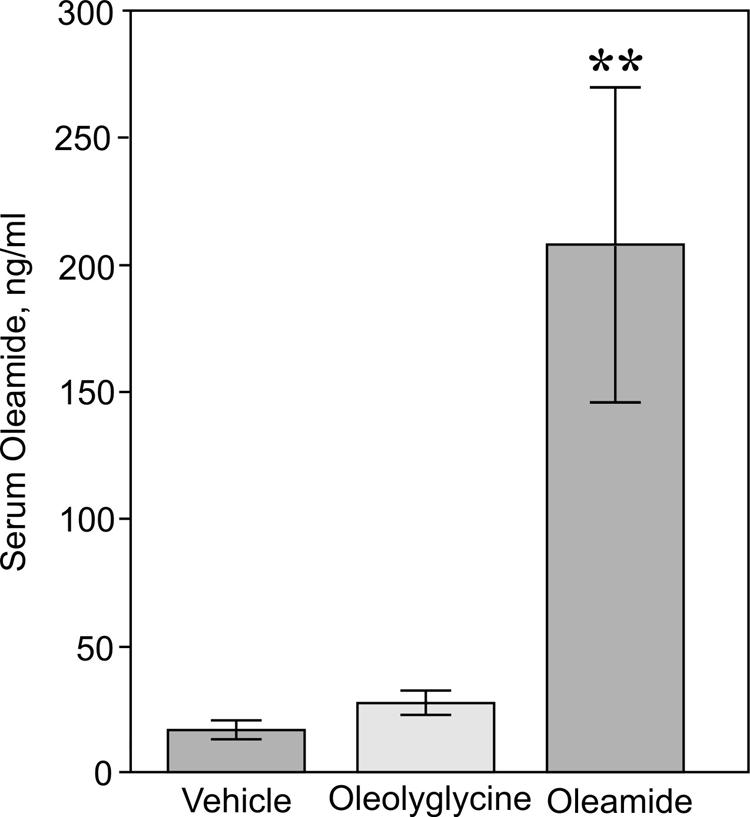
Effects of oleamide and N-oleoylglycine administration on serum levels of oleamide. Groups of rats (N=8) were administered either vehicle, oleoylglycine (80 mg/kg) or oleamide (80 mg/kg) via intraperitoneal injection. After 60 min, the animals were sacrificed, blood samples collected and serum oleamide levels measured by ELISA as described under Materials and Methods. Circulating levels of oleamide were 16 ± 4 ng/ml, 27 ± 6 ng/ml and 209 ± 64 ng/mL for the vehicle control, oleoylglycine and oleamide treatment groups, respectively. * p<0.01
Figure 6.
Dose-response effects of oleamide and oleoylglycine on motor activity in rats. Oleamide or oleoylglycine were administered as single intraperitoneal injections at the doses indicated, and motor activity monitored over the next 60 min. Control animals received injections of vehicle. Data represent accumulated motor activity over the 60 min recording period expressed as a percent of the activity measured in control animals (N=8). * p<0.05; ** p<0.01.
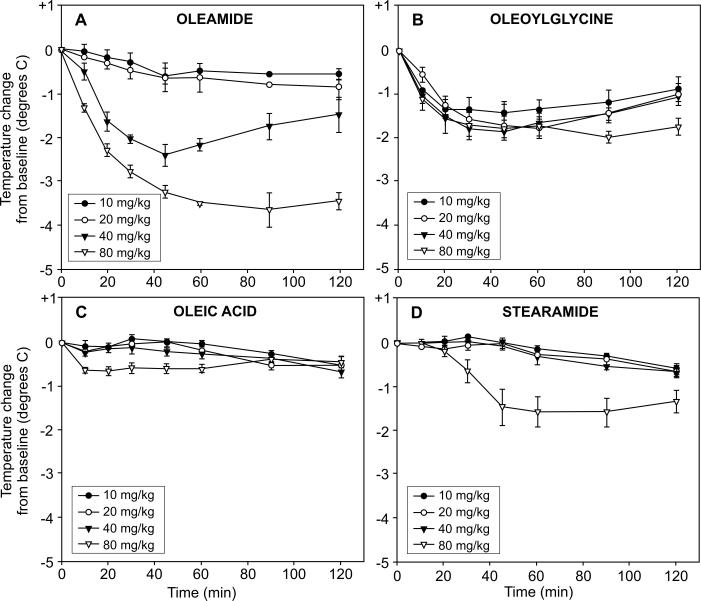
Figure 7 presents the dose-response and time course effects of oleamide administration on core body temperature compared to N-oleoylglycine, oleic acid and stearamide. A single intraperitoneal injection of oleamide decreased core body temperature in a dose- and time-related fashion (Fig. 7, panel A). Reduction of more than 3°C occurred within 60 minutes following the 80 mg/kg dose of oleamide. An intermediate reduction in body temperature occurred following administration of 40 mg/kg oleamide, whereas both the 20 and 10 mg/kg doses had little effect. While the time-course for hypothermia following N-oleoylglycine administration paralleled that oleamide, there was no dose relation observed during the first hour post treatment. All four doses of N-oleoylglycine evoked nearly equal reductions in body temperature (1.5-2.0°C) by 60 minutes post injection (Fig. 7, panel B). A dose-response pattern was somewhat evident at the later time points (100 and 120 min). In contrast to oleamide and N-oleoylglycine, intraperitoneal administration of oleic acid (Fig. 7, panel C) was without effect on core body temperature and stearamide (Fig. 7, panel D) elicited an intermediate response only at the highest dose tested (80 mg/kg).
Figure 7.
Dose-response and time course effects of oleamide, oleoylglycine, oleic acid and stearamide administration on core body temperature in rats. Compounds were administered as single intraperitoneal injections at the doses indicated to groups of rats (N=6). Each animal served as its own baseline control (average of three temperature measurements taken during the 20 min period prior to injection). Core body temperatures were recorded over the next 120 min as shown.
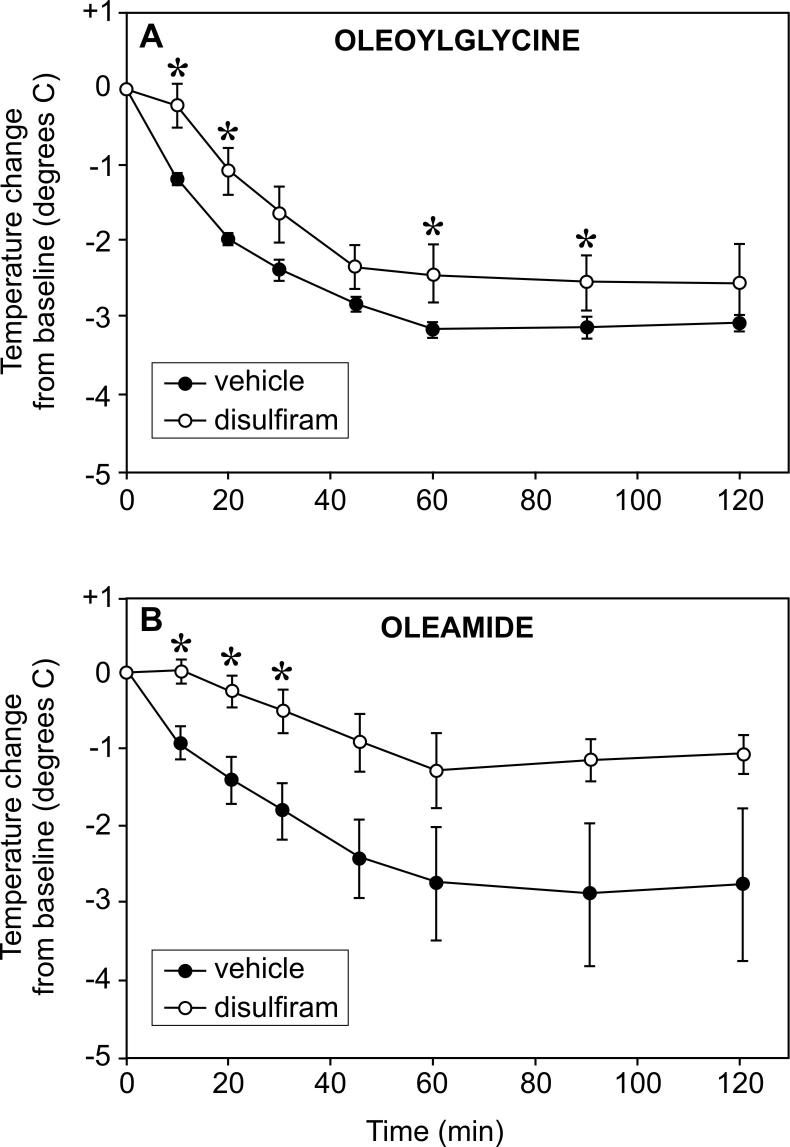
We have previously used the copper chelating compound disulfiram to study the in vivo regulation of PAM activity [25,27]. Here we investigated the effect of pre-treatment with disulfiram on oleamide- and N-oleoylglycine-induced hypothermia. As shown in Figure 8, both oleoylglycine and oleamide decreased body temperature over time. Disulfiram pre-treatment produced modest attenuations in hypothermic effects of both N-oleoylglycine (panel A) and oleamide (panel B) on core body temperature. The statistical significance of these inhibitory effects, however, is limited to the time points indicated by the variance in the data. Inhibition of PAM activity by disulfiram in this investigation was confirmed by measures of 80% - 90% reductions in concentrations of alpha-amidated cholecystokinin in the duodenum, as reported previously (data not shown) [see 25].
Figure 8.
Effects of pre-treatment with disulfiram on oleoylglycine- and oleamide-induced hypothermia. Groups of rats (N=8) received seven daily subcutaneous injections of disulfiram (400 mg/kg, open circles) or vehicle (closed circles) and then challenged 24 h after the last injection with either oleoylglycine (80 mg/kg; panel A) or oleamide (80 mg/kg; panel B). Each animal served as its own baseline control (average of three temperature measurements prior to injection). Core body temperatures were recorded over the next 120 min as shown. * indicates significant difference between vehicle and disulfiram values; p<0.05).
DISCUSSION
Several lines of evidence indicate that oleamide, the prototype primary fatty acid amide, functions as an intercellular messenger mediating the biochemical drive to sleep [5-8]. Despite this intriguing possibility, the biochemical pathway for the synthesis of oleamide remains unknown. The leading hypothesis holds that oleamide is synthesized from oleoylglycine via the actions of the peptide amidating enzyme, peptidylglycine alpha amidating monooxygenase (PAM). The findings presented here indicate that an alternative possibility also exists, that oleoylglycine possesses intrinsic bioactivity that does not depend upon its conversion to oleamide by PAM, or any other mechanism.
A mechanism for oleamide biosynthesis involving oleoylglycine and PAM as the central components predicts that administration of oleoylglycine would increase serum levels of oleamide and reproduce the behavioral responses observed with oleamide, albeit at reduced efficacy and delayed onset, reflecting the efficiency of conversion and time required for PAM to generate oleamide from oleoylglycine. Conversely, pharmacologic inhibition of PAM would prevent the effects of administered oleoylglycine but not those of oleamide. None of these outcomes, however, was clearly observed. While exogenous oleoylglycine was equipotent to oleamide in producing physiologic responses, oleoylglycine failed to increase circulating levels of oleamide as compared to the 8-fold elevation produced by the same dose of oleamide. Further, pretreatment with the established PAM inhibitor, disulfiram [see 17,25,27], which reduced peptide amidation by more than eighty percent, surprisingly produced modest attenuations of the hypothermic effects of both oleoylglycine and oleamide. These modest effects of disulfiram on both oleoylglycine and oleamide responses, and the failure of disulfiram to selectively prevent oleoylglycine-induced hypothermia, suggest that disulfiram mildly interferes with a downstream event that is shared by the actions of both oleamide and oleoylglycine. The present findings that N-oleoylglycine, itself, functions as an intercellular signaling molecule are supported by reports that another long chain fatty acylglycine, arachidonylglycine, is pharmacologically active and occurs naturally in rodent brain and other tissues [28-30]. Accordingly, oleoylglycine and arachidonylglycine may together represent long chain fatty acylglycines as a novel class of signaling molecules.
The failure of oleoylglycine administration to increase serum oleamide concentration was unexpected and suggests that the PAM normally present in the circulation [31,32] may not be enzymatically active within this environment. This conclusion is based upon the assumption that intraperitoneally administered oleoylglycine is absorbed into the circulation, as seen here with oleamide. While peptide alpha-amidation normally occurs within the lumen of secretory granules [17,18], a significant level of PAM protein is present in blood and its activity is readily measurable in vitro in the presence of adequate cofactors: copper, ascorbate and molecular oxygen [17]. Accordingly, inadequate amounts of one or more of these cofactors could be responsible for limiting the catalytic activity of blood-borne PAM. It is possible the PAM has a central role in the intracellular biosynthesis of oleamide such that its actions are not reflected in changes in circulating levels of oleamide. The present findings that oleamide and oleoylglycine shared equivalent dose potencies and time courses of action support the possibility for their independent mechanisms of action.
The biologic importance of PAM in oleamide biosynthesis would be strongly supported by the identification of a robust mechanism for generating N-oleoylglycine. While glycination is important for the excretion of short chain fatty acids [33], detoxification of small-molecule xenobiotics [34,35] and synthesis of bile acids [36,37], the enzymes mediating these conjugations are quite specific and are generally not reactive with long chain fatty acyl-CoAs. Recently, however, O'Byrne and coworkers [22] reported the existence of a bile acid-CoA:amino acid N-acyltransferase (BACAT) that is capable of conjugating glycine to long chain fatty acyl-CoAs, including oleoyl-CoA and arachidonyl-CoA. The enzyme is localized to the cytosol, in contrast to peroxisomes [38,39], and is expressed in brain, kidney and liver [22]. The specific activity of cytosolic BACAT is three- to four-fold higher for saturated C16–C20 acyl-CoAs as compared to their unsaturated counterparts. This fact raises that possibility that a closely related enzyme may exist specifically for the glycination of unsaturated long chain fatty acyl-CoAs. In this regard, sequence analysis of cytosolic BACAT revealed 40-45% homology to a group of enzymes known as the acyl-CoA thioesterase I family [40]. These enzymes have not been characterized with respect to glycine conjugating activity; it is possible that one or more may be shown to effectively catalyze the generation of unsaturated long chain fatty acyl glycines. This demonstration would add support to both the role of PAM in the biosythnesis of unsaturated primary fatty acid amides and also to the possible role of unsaturated long chain fatty acyl glycines as bioactive messengers.
Finally, the present study used a newly development enzyme-linked immunosorbant assay (ELISA) to measure levels of oleamide in serum. This is the first report on the use of an antibody-based assay system to measure oleamide concentrations in a biological sample. The competitive ELISA described here is sensitive to less than 0.05 uM or 14 ng/ml oleamide, and the antibody employed has an absolute requirement for a terminal amide and 9-10 positioning of a double bond for molecular recognition. Accordingly, the antibody does not detect oleic acid and oleoylglycine which terminate in carboxyl groups, or stearamide which lacks the 9-10 double bond. Additionally, erucamide, a twenty-two carbon fatty acid primary amide with a 13-14 double bond does not react with the antibody. Using this ELISA, serum levels of oleamide in vehicle treated rats were estimated to be 16 ± 4 ng/mL, which is in good agreement with the published values of Hanus and coworkers (1999) who used a complex mass spectral analysis procedure to estimate serum oleamide concentrations.
CONCLUSIONS
The present findings suggest that oleoylglycine possesses intrinsic biologic activity which is independent of its conversion to oleamide by PAM. This suggests that oleoylglycine, and possibly long chain fatty acylglycines as a group, function as intercellular messengers. The ability to assay oleamide in biologic samples by ELISA offers a useful technique for investigating the biology of oleamide and oleoylglycine.
Acknowledgements
The authors wish to thank Henry Fales for assisting with the mass spectrometry analyses. We also would like to thank David Merkler and Mitchell Johnson for their helpful discussions on primary fatty acid amides, Betty Eipper and Richard Mains for their discussions on alpha amidation, Adam Uzieblo for his expert technical assistance and synthesis of oleamide 18-aldehyde, Larry Tamarkin for his assistance with the use of colloidal gold in the development of anti-oleamide antibodies, Sarah T. Shafer for her assistance with statistical analyses, and John Fernstrom and Richard Wurtman for their insights into the role of precursor availability in the control neurotransmitter biosynthesis.
REFERENCES
- 1.Boger DL, Henriksen SJ, Cravatt BF. Oleamide: An endogenous sleep-inducing lipid and prototypical member of a new class of biological signaling molecules. Curr Pharm Des. 1998;4:303–14. [PubMed] [Google Scholar]
- 2.Huidobro-Toro JP, Harris RA. Brain lipids that induce sleep are novel modulators of 5-hydroxytrypamine receptors. Proc Natl Acad Sci USA. 1996;93:8078–82. doi: 10.1073/pnas.93.15.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan X, Cravatt BF, Ehring GR, Hall JE, Boger DL, Lerner RA, Gilula NB. The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J Cell Biol. 1997;39:1785–92. doi: 10.1083/jcb.139.7.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boger DL, Patterson JE, Guan X, Cravatt BF, Lerner RA, Gilula NB. Chemical requirements for inhibition of gap junction communication by the biologically active lipid oleamide. Proc Natl Acad Sci USA. 1998;95:4810–15. doi: 10.1073/pnas.95.9.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerner RA, Siuzdak G, Prospero-Garcia O, Henriksen SJ, Boger DL, Cravatt BF. Cerebrodiene: a brain lipid isolated from sleep-deprived cats. Proc Natl Acad Sci USA. 1994;91:9505–8. doi: 10.1073/pnas.91.20.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA. Chemical characterization of a family of brain lipids that induce sleep. Science. 1995;268:1506–9. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- 7.Huitron-Resendiz S, Gombart L, Cravatt BF, Henriksen SJ. Effect of oleamide on sleep and its relationship to blood pressure, body temperature, and locomotor activity in rats. Exp Neurol. 172:235–43. doi: 10.1006/exnr.2001.7792. 2110. [DOI] [PubMed] [Google Scholar]
- 8.Huitron-Resendiz S, Sanchez-Alavez M, Wills DN, Cravatt BF, Henriksen SJ. Characterization of the sleep-wake patterns in mice lacking fatty acid amide hydrolase. Sleep. 2004;27:857–65. doi: 10.1093/sleep/27.5.857. [DOI] [PubMed] [Google Scholar]
- 9.Fedorova I, Hashimoto A, Fecik RA, Hedrick MP, Hanus LO, Boger DL, Rice KC, Basile AS. Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems. J Pharmacol Exp Ther. 2001;299:332–42. [PubMed] [Google Scholar]
- 10.Lichtman AH, Hawkins EG, Griffin G, Cravatt BF. Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J Pharmacol Exp Ther. 2002;302:73–9. doi: 10.1124/jpet.302.1.73. [DOI] [PubMed] [Google Scholar]
- 11.Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100:1375–80. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arafat ES, Trimble JW, Andersen RN, Dass C, Desiderio DM. Identification of fatty acid amides in human plasma. Life Sci. 1989;45:1679–87. doi: 10.1016/0024-3205(89)90278-6. [DOI] [PubMed] [Google Scholar]
- 13.Cravatt BF, Lerner RA, Boger DL. Structure determination of an endogenous sleep-inducing lipid, cis-9-octadecenamide (oleamide): a synthetic approach to the chemical analysis of trace quantities of natural product. J Am Chem Soc. 1996;118:580–90. [Google Scholar]
- 14.Merkler DJ, Merkler KA, Stern W, Fleming FF. Fatty acid amide biosynthesis: a possible new role for peptidylglycine alpha-amidating enzyme and acyl-coenzyme A: glycine Nacyltransferase. Arch Biochem Biophys. 1996;330:430–4. doi: 10.1006/abbi.1996.0272. [DOI] [PubMed] [Google Scholar]
- 15.Sugiura T, Kondo S, Kodaka T, Tonegawa T, Nakane S, Yamashita A, Ishima Y, Waku K. Enzymatic synthesis of oleamide (cis-9, 10-octadecenoamide), an endogenous sleep-inducing lipid, by rat brain microsomes. Biochem Mol Biol Int. 1996;40:931–8. doi: 10.1080/15216549600201553. [DOI] [PubMed] [Google Scholar]
- 16.Bisogno T, Sepe N, De Petrocellis L, Mechoulam R, Di Marzo V. The sleep inducing factor oleamide is produced by mouse neuroblastoma cells. Biochem Biophys Res Commun. 1997;239:473–9. doi: 10.1006/bbrc.1997.7431. [DOI] [PubMed] [Google Scholar]
- 17.Eipper BA, Stoffers DA, Mains RE. The biosynthesis of neuropeptides: Peptide alpha-amidation. Annu Rev Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- 18.Prigge ST, Mains RE, Eipper BA, Amzel LM. New insights into copper monooxygenases and peptide amidation: structure, mechanism and function. Cell Mol Life Sci. 2000;57:1236–59. doi: 10.1007/PL00000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller LA, Baumgart LE, Chew GH, deLong MA, Galloway LC, Jung KW, Merkler KA, Nagle AS, Poore DD, Yoon CH, Merkler DJ. Glutathione, S-substituted glutathiones, and leukotriene C4 as substrates for peptidylglycine alpha-amidating monooxygenase. Arch Biochem Biophys. 2003;412:3–12. doi: 10.1016/s0003-9861(02)00730-0. [DOI] [PubMed] [Google Scholar]
- 20.King L, 3rd, Barnes S, Glufke U, Henz ME, Kirk M, Merkler KA, Vederas JC, Wilcox BJ, Merkler DJ. The enzymatic formation of novel bile acid primary amides. Arch Biochem Biophys. 2000;374:107–17. doi: 10.1006/abbi.1999.1611. [DOI] [PubMed] [Google Scholar]
- 21.Shonsey EM, Sfakianos M, Johnson M, He D, Falany CN, Falany J, Merkler DJ, Barnes S. Bile acid coenzyme A: amino acid N-acyltransferase in the amino acid conjugation of bile acids. Methods Enzymol. 2005;400:374–94. doi: 10.1016/S0076-6879(05)00022-4. [DOI] [PubMed] [Google Scholar]
- 22.O'Byrne J, Hunt MC, Rai DK, Saeki M, Alexson SE. The human bile acid-CoA:amino acid N-acyltransferase functions in the conjugation of fatty acids to glycine. J Biol Chem. 2003;278:34237–44. doi: 10.1074/jbc.M300987200. [DOI] [PubMed] [Google Scholar]
- 23.Merkler DJ, Chew GH, Gee AJ, Merkler KA, Sorondo JP, Johnson ME. Oleic acid derived metabolites in mouse neuroblastoma N18TG2 cells. Biochemistry. 2004;43:12667–74. doi: 10.1021/bi049529p. [DOI] [PubMed] [Google Scholar]
- 24.Hanus LO, Fales HM, Spande TF, Basile AS. A gas chromatographic-mass spectral assay for the quantitative determination of oleamide in biological fluids. Anal Biochem. 1999;270:159–66. doi: 10.1006/abio.1999.4083. [DOI] [PubMed] [Google Scholar]
- 25.Mueller GP, Husten EJ, Mains RE, Eipper BA. Peptide alpha-amidation and peptidylglycine alpha-hydroxylating monooxygenase: Control by disulfiram. Mol Pharmacol. 1993;44:972–80. [PubMed] [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 27.Driscoll WJ, Mueller SA, Eipper BA, Mueller GP. Differential regulation of peptide alpha-amidation by dexamethasone and disulfiram. Mol Pharmacol. 1999;55:1067–76. doi: 10.1124/mol.55.6.1067. [DOI] [PubMed] [Google Scholar]
- 28.Huang SM, Bisogno T, Petros TJ, Chang SY, Zavitsanos PA, Zipkin RE, Sivakumar R, Coop A, Maeda DY, De Petrocellis L, Burstein S, Di Marzo V, Walker JM. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J Biol Chem. 2001;276:42639–44. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- 29.Burstein SH, Huang SM, Petros TJ, Rossetti RG, Walker JM, Zurier RB. Regulation of anandamide tissue levels by N-arachidonylglycine. Biochem Pharmacol. 2002;64:1147–50. doi: 10.1016/s0006-2952(02)01301-1. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda Y, Iguchi H, Nakata M, Ioka RX, Tanaka T, Iwasaki S, Magoori K, Takayasu S, Yamamoto TT, Kodama T, Yada T, Sakurai T, Yanagisawa M, Sakai J. Identification of N-arachidonylglycine, U18666A, and 4-androstene-3,17-dione as novel insulin secretagogues. Biochem Biophys Res Commun. 2005;333:778–86. doi: 10.1016/j.bbrc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Eipper BA, Myers AC, Mains RE. Peptidyl-glycine alpha-amidation activity in tissues and serum of the adult rat. Endocrinology. 1985;116:2497–504. doi: 10.1210/endo-116-6-2497. [DOI] [PubMed] [Google Scholar]
- 32.Kapuscinski M, Green M, Sinha SN, Shepherd JJ, Shulkes A. Peptide alpha-amidation activity in human plasma: relationship to gastrin processing. Clin Endocrinol. 1993;39:51–8. doi: 10.1111/j.1365-2265.1993.tb01750.x. [DOI] [PubMed] [Google Scholar]
- 33.Bonafe L, Troxler H, Kuster T, Heizmann CW, Chamoles NA, Burlina AB, Blau N. Evaluation of urinary acylglycines by electrospray tandem mass spectrometry in mitochondrial energy metabolism defects and organic acidurias. Mol Genet Metab. 2000;69:302–11. doi: 10.1006/mgme.2000.2982. [DOI] [PubMed] [Google Scholar]
- 34.Mawal YR, Qureshi IA. Purification to homogeneity of mitochondrial acyl coa:glycine n-acyltransferase from human liver. Biochem Biophys Res Commun. 1994;205:1373–9. doi: 10.1006/bbrc.1994.2817. [DOI] [PubMed] [Google Scholar]
- 35.Kelley M, Vessey DA. Characterization of the acyl-CoA:amino acid N-acyltransferases from primate liver mitochondria. J Biochem Toxicol. 1994;9:153–8. doi: 10.1002/jbt.2570090307. [DOI] [PubMed] [Google Scholar]
- 36.Kase BF, Prydz K, Bjorkhem I, Pedersen JI. Conjugation of cholic acid with taurine and glycine by rat liver peroxisomes. Biochem Biophys Res Commun. 1986;138:167–73. doi: 10.1016/0006-291x(86)90261-5. [DOI] [PubMed] [Google Scholar]
- 37.Kase BF, Bjorkhem I. Peroxisomal bile acid-CoA:amino-acid N-acyltransferase in rat liver. J Biol Chem. 1989;264:9220–3. [PubMed] [Google Scholar]
- 38.Solaas K, Ulvestad A, Soreide O, Kase BF. Subcellular organization of bile acid amidation in human liver: A key issue in regulating the biosynthesis of bile salts. J Lipid Res. 2000;41:1154–62. [PubMed] [Google Scholar]
- 39.Solaas K, Kase BF, Pham V, Bamberg K, Hunt MC, Alexson SE. Differential regulation of cytosolic and peroxisomal bile acid amidation by PPAR alpha activation favors the formation of unconjugated bile acids. J Lipid Res. 2004;45:1051–60. doi: 10.1194/jlr.M300291-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Hunt MC, Nousiainen SE, Huttunen MK, Orii KE, Svensson LT, Alexson SE. Peroxisome proliferator-induced long chain acyl-CoA thioesterases comprise a highly conserved novel multi-gene family involved in lipid metabolism. J Biol Chem. 1999;274:34317–26. doi: 10.1074/jbc.274.48.34317. [DOI] [PubMed] [Google Scholar]