Abstract
Since their discovery about a decade ago, human Hap46/BAG-1M, the larger isoform Hap50/BAG-1L, and related structures have caused quite some astonishment because of the seemingly unlimited array of possible interaction partners belonging to completely unrelated protein families. This problem was partially resolved when it was realized that molecular chaperones of the heat shock protein family Hsp70 are major primary association partners, which in turn, are able to bind a wide variety of unrelated protein structures, thus forming ternary complexes. Moreover, the protein folding activity of Hsp70 chaperones is affected; hence, the designation “cochaperones.” Although many different proteins require mediation by Hsp70 chaperones for interactions with Hap50/BAG-1L, Hap46/BAG-1M, and isoforms, several other partner proteins are able to associate directly. In addition, Hap46/BAG-1M and Hap50/BAG-1L are also able to interact with DNA by making use of a positively charged region close to the amino terminal end of the polypeptide chain. This is the molecular basis for their effects on transcriptional activities, which are emphasized in this review and for which a molecular model is presented.
DISCOVERY OF BAG-1 AND HAP46/BAG-1M
Roughly a decade has elapsed since the discovery of the intimately related proteins BAG-1 and Hap46/BAG-1M by molecular cloning techniques. This occurred independently in 3 laboratories that were interested in very different biological processes. Takayama et al (1995) searched for interaction partners for the antiapoptotic protein Bcl-2, and they isolated from a mouse embryonic expression library a cDNA coding for a 219–amino acid sequence. In transfection experiments, the protein exhibited inhibitory effects on apoptosis induced by several stimuli and was therefore termed Bcl-2–associated athanogen—in short, BAG-1—with the Greek word athánatos meaning “against death.” The same murine expression library was also used by Bardelli et al (1996), but with the plasma membrane receptor for hepatocyte growth factor as bait protein, leading to the isolation of a series of cDNA clones coding for BAG-1. A third group was interested in binding partners for the activated glucocorticoid receptor (ie, the nuclear form of this intracellular receptor) and employed a human liver cDNA library because liver is a major target organ for glucocorticoids (Zeiner and Gehring 1995). The cDNA cloned in this way encodes a 274-residue protein with a molecular mass of approximately 46 kDa, which was first called RAP46 for “receptor-associating protein of 46-kDa apparent molecular mass.” This designation, however, was subsequently changed to Hap46 (ie, Hsp70/Hsc70-associating protein; Zeiner et al 1997) when it was realized that the heat shock protein of roughly 70 kDa (Hsp70) and the slightly larger constitutively expressed homologue Hsc70 are major interaction partners (see below).
ISOFORMS AND THEIR DOMAIN STRUCTURES
The Bag1 gene is located on human chromosome 9 in region p12 (Takayama et al 1996). It is about 10 kb in size and encompasses 7 exons (see Götz et al 2005). The promoter has been mapped to positions −353 to −54 upstream of the first translational start codon and was found to contain binding sites for several transcription factors (Yang et al 1999). Importantly, p53 mutants derived from human tumors have the ability to upregulate the BAG-1 promoter (Yang et al 1999). The message of roughly 1.5 kb harbors several translation initiation codons, giving rise to a series of polypeptide chains (ie, protein isoforms). The above-mentioned human protein of 274 amino acid residues is synthesized from an AUG codon and thus starts out with methionine (see Fig 1). With cloning of 5′-extended cDNAs of human origin, it became evident that a larger isoform exists, which is generated from an upstream CUG noncanonical start codon, begins with leucine, and is 345 residues long (Packham et al 1997); that is, it contains a 71–amino acid extension at the amino terminal end. This isoform has been found in human and murine cells (Packham et al 1997; Takayama et al 1998; Yang et al 1998), and because the apparent molecular mass is roughly 50 kDa, it has been called p50, Hap50, or BAG-1L (L for long). For the sake of distinction, the human Hap46/BAG-1 was then renamed BAG-1M (M for medium). A characteristic of Hap50/BAG-1L is a series of positively charged residues typical for nuclear localization signals (Fig 1), but Hap46/BAG-1M also contains a cluster of basic amino acids close to its amino terminal end, which is of significance for molecular interactions (see below). Also, a shorter isoform, the 230-residue Hap33/BAG-1S (S for small), originates from an AUG codon further downstream (see Fig 1). In addition, an even shorter form (not included in Fig 1) has been identified that is further truncated amino terminally and consists of 207 amino acids (Yang et al 1998). The level of expression of these isoforms greatly varies among different cell types; however, Hap33/BAG-1S is the predominant form in most cases. Interestingly, there is no direct correlate to human Hap46/BAG-1M in the mouse because the murine message has, instead of the AUG start codon, a GUG in the respective position coding for valine. The originally detected 219-residue murine protein (Takayama et al 1995) is a shorter isoform that is produced from an internal AUG start codon (Townsend et al 2003), which roughly corresponds to human Hap33/BAG-1S. For the sake of distinction, this protein is here being referred to as mouse BAG-1S. Importantly, the human and murine proteins are highly homologous.
Fig 1.
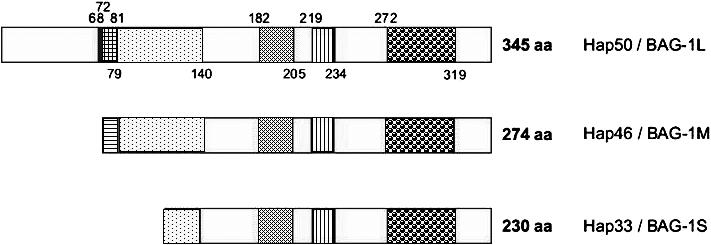
Domain structures of human Hap50/BAG-1L, Hap46/BAG-1M, and Hap33/BAG-1S. Potential nuclear localization signals (vertical hatching) between positions 68 and 79, as well as 219 and 234, in Hap50/BAG-1L. Basic DNA binding domain (horizontal hatching) between positions 72 and 79 in Hap50/BAG-1L (overlap with 1 of the potential nuclear localization signals in Hap50/BAG-1L is indicated by cross-hatching). The sequence is Met-Lys-Lys-Lys-Thr-Arg-Arg-Arg-Ser. Acidic hexarepeat domain between positions 81 and 140 in Hap50/BAG-1L; consensus sequence: Thr-Arg-Ser-Glu-Glu-X. Ubiquitin-like domain between positions 182 and 205 in Hap50/BAG-1L. Hsp70/Hsc70 binding domain = BAG domain between positions 272 and 319 in Hap50/BAG-1L (shown here as originally defined by Takayama et al 1999)
As shown in Figure 1, all isoforms contain the very same carboxy terminal sequence and thus are roughly equivalent in biochemical reactions mediated by this domain (see below). Distinct bioactivities, however, are expected to be produced by greatly differing amino terminal domains. The clusters of basic amino acids within the amino terminal portions of Hap50/BAG-1L and Hap46/BAG-1M are involved in mediating interactions with DNA (see below), whereas isoform Hap33/BAG-1S and mouse BAG-1S do not contain these basic sequences and consequently are devoid of DNA binding ability. The functional significance of the acidic hexarepeat domain (Fig 1) right next to the basic domain is not clear at present, while the ubiquitin-like domain is known to cooperate with the ubiquitin/proteasome machinery of protein degradation (Esser et al 2004). The potential nuclear localization signal roughly in the middle of the sequence (Fig 1) appears to be masked by some unknown mechanism but can become activated with stress conditions because Hap46/BAG-1M accumulates in the nuclear compartment after heat shock (see, eg, Zeiner et al 1999).
INTERACTION WITH HSP70 MOLECULAR CHAPERONES
Human Hap50/BAG-1L, Hap46/BAG-1M, and related proteins have the extraordinary ability to interact with an astounding variety of different protein structures and can, therefore, affect a multitude of bioactivities. At present, it appears, however, that these interactions and activities can be grouped roughly into 3 categories: (1) interactions mediated through Hsp70 molecular chaperones; (2) direct association of Hap50/BAG-1L, Hap46/BAG-1M, and isoforms with several proteins (ie, independence of Hsp70 or Hsc70); and (3) activities of Hap50/BAG-1L and Hap46/BAG-1M mediated through binding to DNA. Combinations of these options could also be of significance, as will be discussed later. A multitude of cellular functions have been listed in previous reviews (Alberti et al 2003; Townsend et al 2003). Here, I will emphasize the effects of Hap50/BAG-1L and Hap46/BAG-1M on transcriptional activities, assuming that these could turn out to be of major physiological relevance.
When various protein-protein interaction assays were used in search of direct binding partners for Hap46/BAG-1M and mouse BAG-1S, only 1 strong signal of about 70 kDa, rather than a multitude of proteins, was obtained with extracts from different cell types; this was easily identified as the molecular chaperone Hsp70/ Hsc70 (Höhfeld and Jentsch 1997; Takayama et al 1997; Zeiner et al 1997). Interestingly, Hsp70 homologues in Saccharomyces cerevisiae, of bacterial or plant origin, and the mammalian endoplasmic form BiP did not bind to Hap46/BAG1M, whereas Hsp70 contained in insect cells was able to interact perfectly well (Zeiner et al 1997).
Preferential interaction with mammalian Hsp70 and Hsc70 immediately raised the question of whether Hap50/BAG-1L, Hap46/BAG-1M, and isoforms might affect protein folding activities. Such effects have indeed been observed by several research groups using different experimental systems (reviewed by Alberti et al 2003; Gehring 2004). The prevalence of interactions with mammalian Hsp70 molecular chaperones is the reason why Hap50/BAG-1L, Hap46/BAG-1M, and isoforms have been called cochaperones. Inasmuch as the effects on the nucleotide exchange reaction of Hsp70/Hsc70 and on protein folding reactions of these chaperones have adequately been summarized by Alberti et al (2003) in this journal, this review rather concentrates on activities beyond these biochemical reactions. Nevertheless, it needs to be mentioned here that these interactions of the cochaperones Hap50/BAG-1L, Hap46/BAG-1M, and isoforms are mediated by the common carboxy terminal region, also called the BAG domain (Fig 1), as has first been demonstrated by deletion of 47 residues from this portion of the molecule (Takayama et al 1997, 1999). On the part of Hsp70 and Hsc70, the ATP binding domain is involved in the interaction, whereas the so-called substrate binding domain remains available for further protein interactions (reviewed by Mayer and Bukau 2005), suggesting the possibility of forming multiple associations. Indeed, ternary complexes have been detected in studies employing various model proteins (Gebauer et al 1997; Takayama et al 1997; Zeiner et al 1997; Bimston et al 1998; Niyaz et al 2003). The mediatory role of Hsp70 chaperones also satisfactorily explains why the use of very different bait proteins in the above-described expression cloning experiments has lead to the identification of the same sequence: Hsc70 contained in these systems served as the interaction mediator. It is interesting to note that variations of the BAG domain also exist in several other proteins that similarly mediate interactions with Hsp70 molecular chaperones and together constitute the BAG protein family (Takayama et al 1999; Takayama and Reed 2001; Sondermann et al 2002).
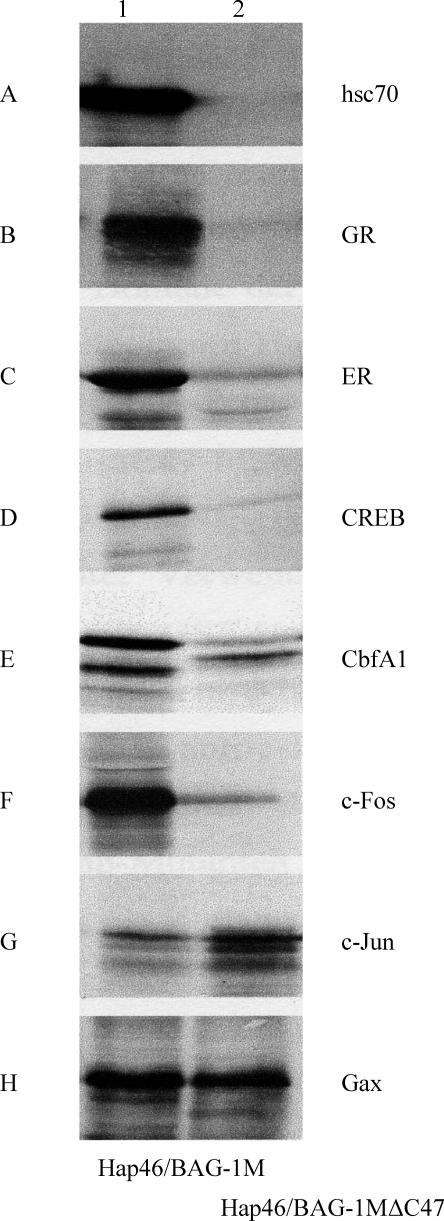
An easy assay for the involvement of Hsp70/Hsc70 in the binding of a given protein to Hap46/BAG-1M is a comparison of pull-down experiments with the full-length protein coupled to a matrix and deletion variant Hap46/BAG-1MΔC47, from which the carboxy terminal 47–amino acid residues have been deleted (Niyaz et al 2003). The matrix containing this deletion variant is unable to retain Hsp70 chaperones (Fig 2A) and consequently does not bind any protein that requires mediation by Hsc70, which is provided in ample amounts with the reticulocyte lysate used for in vitro translation. As shown in Figure 2B–F, this is clearly the case for several transcription factors, like the glucocorticoid and estrogen receptors, both members of the large family of nuclear receptors; the cAMP response element binding protein CREB; the regulator of osteoblast differentiation CbfA1 (also called Runx); and c-Fos, a component of transcription factor AP-1. However, c-Jun, the companion protein of c-Fos in AP1, and Gax, a homeodomain-containing transcription factor involved in the regulation of cell viability, attached in roughly similar extents to both matrices (Fig 2G,H) and apparently do not require the presence of an Hsp70 chaperone as a binding mediator.
Fig 2.
Hap46/BAG-1M forms complexes with various transcription factors. Transcription factors, generated as radiolabeled proteins by in vitro transcription/translation from recombinant constructs, were incubated in the presence of Hsc70, contained in the reticulocyte lysate, with Sepharose matrices to which either Hap46/BAG-1M (lane 1) or deletion variant Hap46/BAG-1MΔC47 (lane 2) were attached. After extensive washing, retained proteins were eluted and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. GR, glucocorticoid receptor; ER, estrogen receptor. (Modified from Niyaz 2003; Niyaz et al 2003)
Hap50/BAG-1L was found to enhance the transactivation function of the androgen receptor, a member of the superfamily of nuclear receptors; however, Hap46/BAG-1M did so only when forced into the nucleus (Knee et al 2001). Although the carboxy terminal Hsp70 binding domain clearly mediates the in vitro interaction, the amino terminal portion of Hap50/BAG-1L is required for nuclear localization and for eliciting the biological effect. This is easily reconciled considering that both the ligand-activated androgen receptor and the cochaperones Hap50/BAG-1L or Hap46/BAG-1M need to be nuclear to cooperate (Knee et al 2001).
Also, the vitamin D receptor, another member of the nuclear receptor family, appears to be somewhat exceptional. Similar to the above-mentioned effect on the androgen receptor, transactivation by the vitamin D receptor was enhanced by Hap50/BAG-1L, but not by shorter, nonnuclear isoforms, and binding was found to depend on the amino terminal part of the molecule. Not just a truncated variant, from which the carboxy terminal BAG domain is missing, but also overexpression of Hap50/ BAG-1L can inhibit vitamin D–dependent transactivation (Guzey et al 2000; Witcher et al 2001), suggesting a regulatory role of this cochaperone in vitamin D action.
DIRECT INTERACTIONS WITH OTHER CELLULAR COMPONENTS
Binding to proteins independent of Hsp70/Hsc70 chaperones
Even though the involvement of Hsp70/Hsc70 in the interaction of many proteins with human Hap50/BAG-1L, Hap46/BAG-1M, Hap33/BAG-1S, or the murine homologues has not been investigated explicitly, direct interaction is unambiguous in several instances. Protein kinase Raf-1 was the first example of a protein that has the ability for direct and specific interaction with the 219-residue mouse BAG-1S, thus leading to increased activity of the kinase (Wang et al 1996). The binding site was mapped adjacent to and partially overlapping that of Hsp70, and specific point mutations within the BAG domain are unable to bind Hsp70 but nevertheless constitutively activate the Raf-1 kinase (Song et al 2001). This then is the molecular basis of competition between Hsp70 and Raf-1 for binding to BAG-1S, which results in an exciting biological effect, namely the coordination of signals for cell growth and mitogenesis. On induction of Hsp70 in response to cell stress, the kinase activity of Raf-1 thus becomes down-regulated (Song et al 2001).
Another very interesting instance of cellular regulation came to light recently when the Bag1 gene was disrupted in mice (Götz et al 2005). Homocygous Bag1−/− embryos that were highly growth-retarded died off before the time of birth and showed multiple developmental defects. In particular, cell survival within the developing nervous and hematopoietic systems was affected because extensive apoptosis occurred in these animals. This again points to the antiapoptotic potential of BAG-1 proteins, as was originally observed by Takayama et al (1995). Even though the activities of protein kinases like Raf/ERK and Akt were not defective in these mutant animals per se, the protein phosphorylation pattern turned out to be affected; in particular, loss of phosphorylation of the regulatory protein Bad at a specific serine residue was observed. This appears to occur by changes in intracellular distributions and targeting, suggesting that at least 1 of the products of the Bag1 gene functions as mediator of extracellular survival signals that normally prevent apoptosis in certain stem cells (Götz et al 2005). It will be interesting to find out to what extent these effects involve Hsp70 molecular chaperones. To this end, it should be helpful to introduce into these mutant animals a Bag1 gene construct from which the region coding for the carboxy terminal BAG domain has been deleted.
Interestingly, a variant of Hap50/BAG-1L has been isolated from a human T-cell cDNA library in a yeast 2-hybrid screen. This turned out to be carboxy terminally truncated by 34 amino acids and harbors alterations within the last stretch of residues (Wadle et al 2005); consequently, it is unable to interact with Hsp70 chaperones. Because this variant was able to bind the tubulin-associating protein RP1, RP1 has to be regarded as another direct interaction partner of Hap50/BAG-1L, as well as Hap46/BAG-1M and other isoforms, thus linking these cochaperones to the microtubule system. Although this deletion variant confers resistance to apoptosis, the details of the interaction still need to be characterized, and perhaps more importantly, the molecular consequences on the cytoskeleton, cellular mobility, and cytokinesis will be of interest.
Siah-1A, a human homologue of Drosophila seven in absentia, was also identified in yeast 2-hybrid tests as a binding partner of mouse BAG-1S and similarly reacted positively in other protein-protein assays (Matsuzawa et al 1998). In this case, however, the situation appears less clear because a carboxy terminally truncated variant failed to bind Siah-1A, suggesting at least some involvement of Hsp70/Hsc70.
Among transcription factors in the broad sense, the retinoblastoma susceptibility protein Rb needs to be mentioned as another direct binding partner of Hap46/BAG-1M because it is able to interact with mutants defective in Hsp70/Hsc70 binding (Arhel et al 2003). The complex between Rb and Hap46/BAG-1M is stable enough that Hap46/BAG-1M is cotransported by Rb into the nucleus and specificity is underlined by the observation that the complex is disrupted by the human papilloma virus oncoprotein E7, an inhibitor of Rb protein interactions (Arhel et al 2003). However, the interacting regions on both partner proteins need to be further characterized. Nevertheless, this modulation of subcellular localization of Hap46/BAG-1M has significant consequences for the survival of colorectal epithelial and tumor cells (Clemo et al 2005) and suggests that the Rb protein is a physiological regulator of Hap46/BAG-1M.
Interaction with DNA
Even though Hap46/BAG-1M as a whole is acidic in nature with pI 5.3, it contains a prominent cluster of basic amino acids immediately adjacent to the amino terminal end of the polypeptide chain (Takayama et al 1996). This region was then identified to be involved in binding to DNA. Interestingly, the DNA could be in a linear or a supercoiled state, and neither the origin of the DNA nor its size appear to be of any significance, except that a minimum length is required for this interaction (Zeiner et al 1999; Niyaz et al 2003). Hap46/BAG-1M has no nucleotide sequence specificity, as became clear when a series of overlapping oligonucleotides was investigated that cover the entire early promotor region of the cytomegalovirus (Niyaz et al 2003). This nonspecific DNA binding ability, however, completely depends on the positively charged sequence at the amino terminus, which consists of 2 groups of 3 consecutive lysines and of 3 arginines, separated by a centrally located neutral residue (see legend to Fig 1). Mutation analysis has established that both of these trimeric blocks contribute to the DNA binding ability of Hap46/BAG-1M (Zeiner et al 1999; Schmidt et al 2003) and that changing the spacing between them had no effect (Schmidt et al 2003). In Hap50/BAG-1L, the same basic peptide region (see Fig 1) is available for interaction with DNA, but the polypeptide chain is extended farther at the amino terminal end. Interestingly, the above nonspecific DNA binding motif is involved in down-regulation of glucocorticoid receptor–mediated transactivation by Hap46/BAG-1M (Schneikert et al 2000; Schmidt et al 2003).
In the context of this review, it is of interest to note that both major interaction regions located toward the extreme ends of the amino acid sequences of Hap46/BAG-1M and Hap50/BAG-1L (ie, for DNA binding and for interaction with Hsp70 chaperones) are simultaneously available for binding reactions. Thus, trimeric complexes of the type DNA·Hap46/BAG-1M·Hsc70 can be formed (Zeiner et al 1999; Niyaz et al 2003) in which the substrate binding domain of Hsc70 still remains available for further interactions with other proteins, for example transcription factors.
EFFECTS OF HAP46/BAG-1M AND HAP50/BAG-1L ON TRANSCRIPTION
The above-mentioned observation of DNA binding immediately prompted a search for effects of Hap46/BAG-1M on transcription. This suggestion is corroborated by the finding that under heat stress conditions, a significant portion of Hap46/BAG-1M translocates from the cytoplasm to the cell nucleus (Zeiner et al 1999). By contrast, Hap50/BAG-1L is largely a nuclear protein to begin with (Packham et al 1997; Takayama et al 1998; Knee et al 2001; Niyaz et al 2001), suggesting some specific biological role of this isoform in the nucleus. In vitro transcription assays with HeLa nuclear extracts indeed showed a significant stimulation (roughly 10-fold) of transcription from various templates on addition of Hap46/BAG-1M (Zeiner et al 1999; Niyaz et al 2003). It did not matter whether eukaryotic or prokaryotic DNA was used as template and whether promoter elements were present or not. The transcripts have no distinct length, suggesting random initiation and termination of transcription at the end of linearized DNA fragments. Involvement of the amino terminal DNA binding region in the effect on transcription became evident from the observation that deletion of the 10 amino terminal residues from Hap46/BAG-1M resulted in complete loss of stimulation (Zeiner et al 1999).
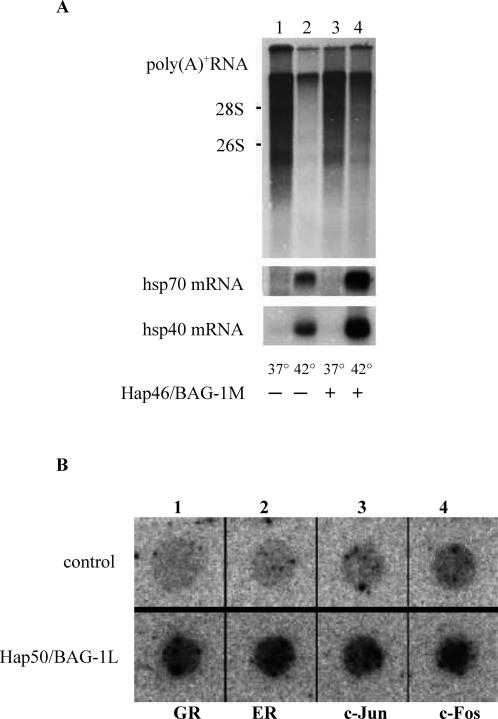
These in vitro studies were complemented by experiments with intact cells that were transfected with an expression vector for Hap46/BAG-1M. On exposing these cells to heat shock that causes nuclear translocation, as mentioned above, overexpression of Hap46/BAG-1M largely compensated the drastic shutdown of cellular transcription that normally occurs after heat stress (Fig 3A, upper panel, lane 4 vs 2). Interestingly, induction of heat shock proteins Hsp70 and Hsp40 was further stimulated by Hap46/BAG-1M (Fig 3A, lower panels, lane 4 vs 2). With overexpression of Hap50/BAG-1L in a similar cell system, increased transcriptional activities were again observed. Predominantly endogenous genes that are under cellular regulation (eg, those encoding c-Jun, c-Fos, or the receptors for glucocorticoids or estrogens) were subject to stimulation (Fig 3B), whereas the expression of actin, for example, was not accelerated further (Niyaz et al 2001).
Fig 3.
Stimulation of transcription by Hap46/BAG-1M and Hap50/ BAG-1L. (A) Human DU145 cells were transfected with an expression vector encoding Hap46/BAG1M (lanes 3, 4) or not (lanes 1, 2). As indicated, cells were submitted to heat shock for 2 hours at 42°C. Newly synthesized poly(A)+ RNA was analyzed by gel electrophoresis and autoradiography (upper panel), the intensity of blackening giving a measure for the stimulation of transcription. The positions of 28S and 18S rRNAs are shown along the margin. The same filters were subsequently used for Northern hybridization with labeled cDNAs for Hsp70 and Hsp40 (lower panels) (from Zeiner et al 1999). (B) Human HeLa cells were transfected with an expression vector encoding Hap50/BAG-1L (lower panel) or not (upper panel) and used for nuclear runoff transcription assays. Labeled RNAs were probed with dot-blotted cDNAs for GR (= glucocorticoid receptor), ER (= estrogen receptor), c-Jun, or c-Fos (from Niyaz et al 2001)
A molecular model for the effects of Hap46/BAG-1M and Hap50/BAG-1L on transcription, as put together in Figure 4, has to take into account 2 prominent features of these cochaperones: DNA binding ability and the potential to interact with a variety of transcription factors either directly or by mediation through Hsp70 chaperones. As depicted in Figure 4, several molecules of Hap46/BAG-1M might bind simultaneously to a stretch of nucleosome-free DNA within a region of transcriptionally active chromatin, making them available for establishing contact with the transcriptional apparatus itself, or with transcription factors attached to DNA at quite distant locations, or with both. In this way, Hap46/BAG-1M could help recruit various protein components and assemble them into functional transcriptional complexes, eventually leading to increased expression of the specific gene. The very same considerations also apply to the large isoform Hap50/BAG-1L. A transcription factor attached to Hap46/BAG1M, as shown in Figure 4, could represent, for example, a steroid hormone receptor that in turn is bound to its specific response element on the DNA upstream of the gene to be transcribed. It is interesting in this context that Hap50/BAG-1L was found to increase estrogen-dependent transcription in breast cancer cells and thus is an important determinant of estrogen receptor function (Cutress et al 2003). Several transcription factors might be in contact through neighboring molecules of Hap46/BAG-1M or Hap50/BAG-1L attached to DNA in juxtaposition (not included in Fig 4). In fact, chromatin immunoprecipitation studies, in which the cochaperone Hap50/BAG-1L, the chaperone Hsp70, and the androgen receptor were found targeted to the relevant response element, support the above notion (Shatkina et al 2003) and further suggest nucleation of transcriptional regulatory complexes.
Fig 4.
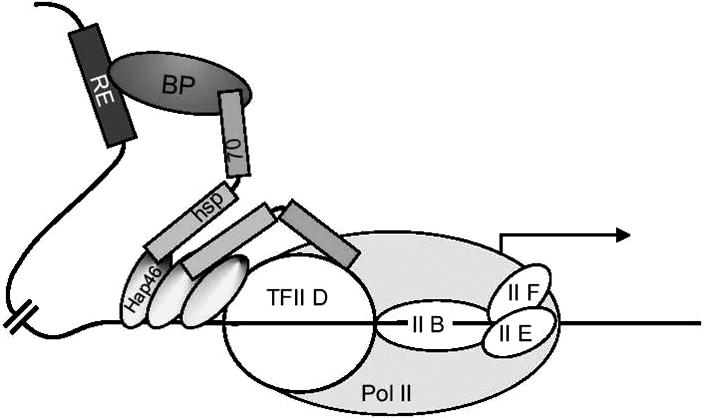
Molecular model for the chaperoning of transcription factors by Hap46/BAG-1M. A simplified version of the transcriptional initiation complex is shown arbitrarily with 3 molecules of Hap46/BAG-1M bound to DNA; these are shown to establish various contacts via Hsp70 chaperones. Pol II, RNA polymerase II; TFII, basal transcription factor II; RE, response element on DNA; BP, specific response element binding protein, for example, a steroid hormone receptor complexed with its specific ligand (from Gehring 2004)
The effects of the cochaperones Hap46/BAG-1M and Hap50/BAG-1L on transcription, however, do not necessarily need to be positive, and in case of steroid hormone receptors as transcriptional regulators, the responses are not at all uniform, as has been pointed out by Cato and Mink (2001). The inhibitory activity of Hap46/BAG-1M on glucocorticoid receptor-mediated transcription was found to depend on the amino terminal DNA binding region, and Hap33/BAG-1S is unable to elicit this effect. In addition, an intact carboxy terminal domain for interaction with Hsp70 chaperones is also required (Schneikert et al 2000; Schmidt et al 2003); that is, both functional regions close to the ends of the Hap46/BAG-1M sequence need to be present in cis. The mechanistic details of these interactions, however, remain somewhat vague at present.
FURTHER DIRECTIONS OF RESEARCH
The above-described molecular model of Figure 4 suggests a multitude of details that need to be checked carefully and elucidated. In particular, it will be interesting to find out how various transcription factors cooperate molecularly with Hap46/BAG-1M and Hap50/BAG-1L to bring about specific effects. Most likely, in addition to the tumor suppressor protein Rb, c-Jun, and Gax, other transcription factors will be identified to interact directly with the transcription-stimulating protein Hap46/BAG-1M (ie, independent of Hsp70 molecular chaperones).
In the case of AP-1, the situation is particularly interesting because 1 component, c-Fos, requires mediation by an Hsp70 chaperone, whereas the other, c-Jun, is able to interact directly with Hap46/BAG-1M (see Fig 2). This difference between c-Fos and c-Jun is interesting in view of the similarities these proteins exhibit in domain structure and their ability to form bioactive heterodimers. Modulation of the transcriptional effects of c-Jun and Hap46/BAG-1M have indeed been observed in transfected cells (I. Michel and G. Petersen, unpublished data); however, these experiments need to be further elaborated. Also, the respective interaction regions need to be delineated. It is worth noting in this context that the DNA binding characteristics of AP-1 are altered by Hsp70 heat shock proteins (Carter 1997). This certainly supports the notion that the DNA binding proteins Hap46/BAG-1M and AP-1, in conjunction with Hsp70 molecular chaperones, participate in forming a network of protein-protein and protein-DNA interactions that might be involved in targeting and activating the basal transcription machinery of the cell.
Although addition of Hap46/BAG-1M to the in vitro transcription system clearly caused a rather large positive effect (see above), it came as a surprise that the carboxy terminal deletion variant Hap46/BAG-1MΔC47 also produced some, albeit moderate, stimulation (Niyaz et al 2003). This observation clearly points to the involvement of Hsp70 or Hsc70 and suggests that Hsp70 molecular chaperones themselves play an important role in transcription, even though the molecular mechanisms involved still need to be characterized. The importance of chaperones in the modeling of transcriptional complexes has also come to the attention of other authors (Morimoto 2002; Freeman and Yamamoto 2002). In this context, it is of significance that newly produced Hsp70 during environmental stress readily becomes nuclear, as had been established some time ago (Velazquez and Lindquist 1984). Because the cochaperone Hap46/BAG-1M similarly accumulates in the cell nucleus in response to heat shock, cooperation between these proteins to affect transcription is an attractive possibility, as delineated in Figure 4.
By contrast, the prominently expressed short isoform Hap33/BAG-1S, which is devoid of DNA binding ability, appears most suitable for eliciting effects on protein folding or degradation. Because multiple biochemical reactions possibly cooperate in eliciting complex cellular responses, it is conceivable that modulation of transcription by Hap50/BAG-1L or Hap46/BAG-1M, as well as the effects of Hap33/BAG-1S on refolding or degradation of misfolded proteins, might contribute to the repulse of apoptosis. It will be of interest to clarify the shares of these individual reactions. Moreover, cellular control of expression of the different isoforms needs to be investigated, which could depend, at least to some extent, on cap-independent initiation of translation through internal ribosome entry sites (Han and Zhang 2002; Pickering et al 2003). Interestingly, stage- and site-specific expression of isoforms has been observed during mouse development (Crocoll et al 2000).
As mentioned in this paper, Hap50/BAG-1L, Hap46/ BAG-1M, and isoforms play a significant role in modulating cell survival, which is of particular importance in neoplastic conditions and under cell stress. During recent years, these cochaperones became markers for the aggressiveness of cancer cells and, consequently, for the clinical prognosis of patients. Such correlations have been observed in many clinical and cell culture studies (see, eg, Takayama et al 1998; Cutress et al 2002; Kudoh et al 2002; Tang 2002; Pusztai et al 2004; Tang et al 2004; Townsend et al 2005; Krajewska et al 2006), which could be viewed in the context that expression of the Bag1 gene is enhanced by tumor-derived p53 mutants (Yang et al 1999). In particular, Hap50/BAG-1L is often expressed at rather high levels in various tumors compared with normal cells. Even more importantly, the cochaperones Hap50/BAG-1L and Hap46/BAG-1M could serve in the future as molecular targets for therapeutic approaches, as has been discussed, for example, in reviews by Gehring (2004), Sharp et al (2004), and Townsend et al (2005), and it will be very helpful for the treatment of some cancer types if the cellular levels of Hap50/BAG-1L and Hap46/ BAG-1M could be lowered by specific drugs. In addition, molecular chaperones themselves play a role in carcinogenesis and are hoped to soon become available for pharmacological interventions (Jäättelä 1999; Mosser and Morimoto 2004).
REFERENCES
- Alberti S, Esser C, Höhfeld J. BAG-1—a nucleotide exchange factor of Hsc70 with multiple cellular functions. Cell Stress Chaperones. 2003;8:225–231. doi: 10.1379/1466-1268(2003)008<0225:bnefoh>2.0.co;2.1466-1268(2003)008[0225:BNEFOH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arhel NJ, Packham G, and Townsend PA. et al. 2003 The retinoblastoma protein interacts with Bag1 in human colonic adenoma and carcinoma derived cell lines. Int J Cancer. 106:364–371. [DOI] [PubMed] [Google Scholar]
- Bardelli A, Longati P, Albero D, Goruppi S, Schneider C, Ponzetto C, Comoglio PM. HGF receptor associates with the anti-apoptotic protein BAG-1 and prevents cell death. EMBO J. 1996;15:6205–6212.1460-2075(1996)015[6205:HRAWTA]2.0.CO;2 [PMC free article] [PubMed] [Google Scholar]
- Bimston D, Song J, Winchester D, Takayama S, Reed JC, Morimoto RI. BAG-1, a negative regulator of Hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J. 1998;17:6871–6878. doi: 10.1093/emboj/17.23.6871.1460-2075(1998)017[6871:BANROH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DA. Modulation of cellular AP-1 DNA binding activity by heat shock proteins. FEBS Lett. 1997;416:81–85. doi: 10.1016/s0014-5793(97)01174-5.0014-5793(1997)416[0081:MOCADB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cato AC, Mink S. BAG-1 family of cochaperones in the modulation of nuclear receptor action. J Steroid Biochem Mol Biol. 2001;78:379–388. doi: 10.1016/s0960-0760(01)00114-5.0960-0760(2001)078[0379:BFOCIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Clemo NK, Arhel NJ, Barnes JD, Baker J, Moorghen M, Packham GK, Paraskeva G, Williams AC. The role of the retinoblastoma protein (Rb) in the nuclear localization of BAG-1: implications for colorectal tumour cell survival. Biochem Soc Trans. 2005;33(4):676–678. doi: 10.1042/BST0330676.0300-5127(2005)033[0676:TROTRP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Crocoll A, Blum M, Cato AC. Isoform-specific expression of BAG-1 in mouse dvelopment. Mech Dev. 2000;91:355–359. doi: 10.1016/s0925-4773(99)00284-1.1872-6356(2000)091[0355:IEOBIM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cutress RI, Townsend PA, Brimmell M, Bateman AC, Hague A, Packham G. BAG-1 expression and function in human cancer. Br J Cancer. 2002;87:834–839. doi: 10.1038/sj.bjc.6600538.0007-0920(2002)087[0834:BEAFIH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutress RI, Townsend PA, and Sharp A. et al. 2003 The nuclear BAG-1 isoform, BAG-1L, enhances oestrogen-dependent transcription. Oncogene. 22:4973–4982. [DOI] [PubMed] [Google Scholar]
- Esser C, Alberti S, Höhfeld J. Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochim Biophys Acta. 2004;1695:171–188. doi: 10.1016/j.bbamcr.2004.09.020.0006-3002(2004)1695[0171:COMCWT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Freeman BC, Yamamoto KR. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science. 2002;296:2232–2235. doi: 10.1126/science.1073051.0193-4511(2002)296[2232:DOTRCB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gebauer M, Zeiner M, Gehring U. Proteins interacting with the molecular chaperone Hsp70/Hsc70: physical associations and effects on refolding activity. FEBS Lett. 1997;417:109–113. doi: 10.1016/s0014-5793(97)01267-2.0014-5793(1997)417[0109:PIWTMC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gehring U. Biological activities of HAP46/BAG-1. EMBO Rep. 2004;5:148–153. doi: 10.1038/sj.embor.7400083.1469-221X(2004)005[0148:BAOB]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz R, Wiese S, and Takayama S. et al. 2005 Bag1 is essential for differentiation and survival of hematopoietic and neuronal cells. Nat Neurosci. 8:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzey M, Takayama S, Reed JC. BAG-1L enhances trans-activation function of the vitamin D receptor. J Biol Chem. 2000;275:40 749–40756. doi: 10.1074/jbc.M004977200.0021-9258(2000)275[40 749:BETFOT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Han B, Zhang JT. Regulation of gene expression by internal ribosome entry sites or cryptic promotors: the eIF4G story. Mol Cell Biol. 2002;22:7372–7384. doi: 10.1128/MCB.22.21.7372-7384.2002.0270-7306(2002)022[7372:ROGEBI]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhfeld J, Jentsch S. GrpE-like regulation of the Hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 1997;16:6209–6216. doi: 10.1093/emboj/16.20.6209.1460-2075(1997)016[6209:GROTHC]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäättelä M. Escaping cell death: survival proteins in cancer. Exp Cell Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455.0014-4827(1999)248[0030:ECDSPI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Knee DA, Froesch BA, Nuber U, Takayama S, Reed JC. Structure-function analysis of Bag1 proteins. Effects on androgen receptor transcriptional activity. J Biol Chem. 2001;276:12718–12724. doi: 10.1074/jbc.M010841200.0021-9258(2001)276[12718:SAOBPE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Krajewska M, Turner BC, Shabaik A, Krajewski S, Reed JC. Expression of BAG-1 protein correlates with aggressive behavior of prostate cancers. Prostate. 2006;66:801–810. doi: 10.1002/pros.20384.0270-4137(2006)066[0801:EOBPCW]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kudoh M, Knee DA, Takayama S, Reed JC. Bag1 proteins regulate growth and survival of ZR-75-1 human breast cancer cells. Cancer Res. 2002;62:1904–1909.0008-5472(2002)062[1904:BPRGAS]2.0.CO;2 [PubMed] [Google Scholar]
- Matsuzawa S, Takayama S, Froesch BA, Zapata JM, Reed JC. p53-Inducible human homologue of Drosophila seven in absentia (Siah) inhibits cell growth: suppression by BAG-1. EMBO J. 1998;17:2736–2747. doi: 10.1093/emboj/17.10.2736.1460-2075(1998)017[2736:PHHODS]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6.1420-682X(2005)062[0670:HCCFAM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Dynamic remodeling of transcription complexes by molecular chaperones. Cell. 2002;110:281–284. doi: 10.1016/s0092-8674(02)00860-7.0092-8674(2002)110[0281:DROTCB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23:2907–2918. doi: 10.1038/sj.onc.1207529.0950-9232(2004)023[2907:MCATSO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Niyaz Y 2003. Funktionelle Charakterisierung des humanen Hsc70/ Hsp70-assoziierenden Proteins Hap46 und seiner Isoform Hap50. PhD thesis, Ruprecht-Karls-Universität Heidelberg, Germany. [Google Scholar]
- Niyaz Y, Frenz I, Petersen G, Gehring U. Transcriptional stimulation by the DNA binding protein Hap46/BAG-1M involves Hsp70/Hsc70 molecular chaperones. Nucleic Acids Res. 2003;31:2209–2216. doi: 10.1093/nar/gkg303.0305-1048(2003)031[2209:TSBTDB]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyaz Y, Zeiner M, Gehring U. Transcriptional activation by the human Hsp70-associating protein Hap50. J Cell Sci. 2001;114:1839–1845. doi: 10.1242/jcs.114.10.1839.0021-9533(2001)114[1839:TABTHH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Packham G, Brimmel M, Cleveland JL. Mammalian cells express two differently localized Bag-1 isoforms generated by alternative translation initiation. Biochem J. 1997;328:807–813. doi: 10.1042/bj3280807.0264-6021(1997)328[0807:MCETDL]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering BM, Mitchell SA, Evans JR, Willis AE. Polypyrimidine tract binding protein and poly r(C) binding protein 1 interact with the BAG-1 IRES and stimulate its activity in vitro and in vivo. Nucleic Acids Res. 2003;31:639–646. doi: 10.1093/nar/gkg146.0305-1048(2003)031[0639:PTBPAP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai L, Krishnamurti S, and Perez Cardona J. et al. 2004 Expression of BAG-1 and Bcl-2 proteins before and after neoadjuvant chemotherapy of locally advanced breast cancer. Cancer Invest. 22:248–256. [DOI] [PubMed] [Google Scholar]
- Shatkina L, Mink S, Rogatsch H, Klocker H, Langer G, Nestl A, Cato AC. The cochaperone Bag-1L enhances androgen receptor action via interaction with the NH2-terminal region of the receptor. Mol Cell Biol. 2003;23:7189–7197. doi: 10.1128/MCB.23.20.7189-7197.2003.0270-7306(2003)023[7189:TCBEAR]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt U, Wochnik GM, Rosenhagen MC, Young JC, Hartl FU, Holsboer F, Rein T. Essential role of the unusual DNA-binding motif of BAG-1 for inhibition of the glucocorticoid receptor. J Biol Chem. 2003;278:4926–4931. doi: 10.1074/jbc.M212000200.0021-9258(2003)278[4926:EROTUD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schneikert J, Hübner S, Langer G, Petri T, Jäättelä M, Reed J, Cato ACB. Hsp70-RAP46 interaction in downregulation of DNA binding by glucocorticoid receptor. EMBO J. 2000;19:6508–6516. doi: 10.1093/emboj/19.23.6508.1460-2075(2000)019[6508:HIIDOD]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A, Crabb SJ, Cutress RI, Brimmell M, Wang XH, Packham G, Townsend PA. BAG-1 in carcinogenesis. Expert Rev Mol Med. 2004;2004:1–15. doi: 10.1017/S1462399404007537.1462-3994(2004)2004[0001:BIC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sondermann H, Ho AK, Listenberger LL, Siegers K, Moarefi I, Wente SR, Hartl F-U, Young JC. Prediction of novel Bag-1 homologs based on structure/function analysis identifies Snl1p as an Hsp70 co-chaperone in Saccharomyces cerevisiae. J Biol Chem. 2002;277:33220–33227. doi: 10.1074/jbc.M204624200.0021-9258(2002)277[33220:PONBHB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Song J, Takeda M, Morimoto RI. Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nature Cell Biol. 2001;3:276–282. doi: 10.1038/35060068.1465-7392(2001)003[0276:BMAPSS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, Morimoto RI, Reed JC. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887.1460-2075(1997)016[4887:BMTCAO]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Kochel K, and Irie S. et al. 1996 Cloning of cDNAs encoding the human BAG1 protein and localization of the human BAG1 gene to chromosome 9p12. Genomics. 35:494–498. [DOI] [PubMed] [Google Scholar]
- Takayama S, Krajewski S, and Krajewska M. et al. 1998 Expression and location of Hsp70/Hsc-binding anti-apoptotic protein BAG-1 and its variants in normal tissues and tumor cell lines. Cancer Res. 58:3116–3131. [PubMed] [Google Scholar]
- Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nature Cell Biol. 2001;3:E237–E241. doi: 10.1038/ncb1001-e237.1465-7392(2001)003[E237:MCTARB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Takayama S, Sato T, Krajewski S, Kochel K, Irie S, Millan JA, Reed JC. Cloning and functional analysis of BAG-1: a novel Bcl-2–binding protein with anti–cell death activity. Cell. 1995;80:279–284. doi: 10.1016/0092-8674(95)90410-7.0092-8674(1995)080[0279:CAFAOB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. doi: 10.1074/jbc.274.2.781.0021-9258(1999)274[0781:AECFOH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tang SC. BAG-1, an anti-apoptotic tumour marker. IUBMB Life. 2002;53:99–105. doi: 10.1080/15216540211473.1521-6543(2002)053[0099:BAATM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tang SC, Beck J, Murphy S, Chernenko G, Robb D, Watson P, Khalifa M. BAG-1 expression correlates with Bcl-2, p53, differentiation, estrogen and progesterone receptors in invasive breast carcinoma. Breast Cancer Res Treat. 2004;84:203–213. doi: 10.1023/B:BREA.0000019951.32001.93.0167-6806(2004)084[0203:BECWBP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Townsend PA, Cutress RI, Sharp A, Brimmell M, Packham G. BAG-1: a multifunctional regulator of cell growth and survival. Biochim Biophys Acta. 2003;1603:83–98. doi: 10.1016/s0304-419x(03)00002-7.0006-3002(2003)1603[0083:BAMROC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Townsend PA, Stephanou A, Packham G, Latchman DS. BAG-1: a multi-functional pro-survival molecule. Int J Biochem Cell Biol. 2005;37:251–259. doi: 10.1016/j.biocel.2004.03.016.1357-2725(2005)037[0251:BAMPM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Velazquez JM, Lindquist S. Hsp70: nuclear concentration during environmental stress and cytoplasmatic storage during recovery. Cell. 1984;36:655–662. doi: 10.1016/0092-8674(84)90345-3.0092-8674(1984)036[0655:HNCDES]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wadle A, Mischo A, and Henrich PP. et al. 2005 Characterization of Hap/BAG-1 variants as RP1 binding proteins with antiapoptotic activity. Int J Cancer. 117:896–904. [DOI] [PubMed] [Google Scholar]
- Wang H-G, Takayama S, Rapp UR, Reed JC. Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc Natl Acad Sci USA. 1996;93:7063–7068. doi: 10.1073/pnas.93.14.7063.1091-6490(1996)093[7063:BIPBBT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcher M, Yang X, Pater A, Tang S-C. BAG-1 p50 isoform interacts with the vitamin D receptor and its cellular overexpression inhibits the vitamin D pathway. Exp Cell Res. 2001;265:167–173. doi: 10.1006/excr.2001.5176.0014-4827(2001)265[0167:BPIIWT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yang X, Chernenko G, Hao Y, Ding Z, Pater MM, Pater A, Tang S-C. Human BAG1/RAP46 protein is generated as four isoforms by alternative translation initiation and overexpressed in cancer cells. Oncogene. 1998;17:981–989. doi: 10.1038/sj.onc.1202032.0950-9232(1998)017[0981:HRPIGA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yang X, Pater A, Tang SC. Cloning and characterization of the human BAG-1 gene promoter: upregulation by tumor-derived p53 mutants. Oncogene. 1999;18:4546–4553. doi: 10.1038/sj.onc.1202843.0950-9232(1999)018[4546:CACOTH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zeiner M, Gehring U. A protein that interacts with members of the nuclear hormone receptor family: identification and cDNA cloning. Proc Natl Acad Sci USA. 1995;92:11465–11469. doi: 10.1073/pnas.92.25.11465.1091-6490(1995)092[11465:APTIWM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiner M, Gebauer M, Gehring U. Mammalian protein RAP46: an interaction partner and modulator of 70 kDa heat shock proteins. EMBO J. 1997;16:5483–5490. doi: 10.1093/emboj/16.18.5483.1460-2075(1997)016[5483:MPRAIP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiner M, Niyaz Y, Gehring U. The hsp70-associating protein Hap46 binds to DNA and stimulates transcription. Proc Natl Acad Sci USA. 1999;96:10194–10199. doi: 10.1073/pnas.96.18.10194.1091-6490(1999)096[10194:THPHBT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]