Abstract
Heat shock protein 72 (Hsp72) has been detected in the peripheral circulation of humans. Because intracellular Hsp72 binds to aggregated proteins, we hypothesized that postexercise plasma-derived Hsp72 concentrations would be greater than serum-derived Hsp72 because of binding of Hsp72 to aggregated clotting proteins in serum. Postexercise serum, heparin, and ethylenediaminetetraacetic acid (EDTA) samples were collected from 9 recreationally active males and were analyzed for Hsp72 by enzyme-linked immunosorbent assay. In line with our hypothesis, EDTA-treated blood was significantly higher in Hsp72 concentration than all other treatments (P ≤ 0.001), whilst heparin plasma (LH) was significantly higher than serum derived on ice (SI) and at room temperature (SR) (P < 0.05; EDTA: 6.46 ± 0.76, LH: 2.73 ± 2.26, SI: 0.13 ± 0.24, SR: 0.20 ± 0.32 ng/mL). Because previous research has tended to report serum data at the lowest point of the detectable range of the assay, it is recommended that EDTA specimen tubes be used in future investigations.
INTRODUCTION
The 70-kDa family of heat shock proteins (HSP70) perform many intracellular functions vital to the maintenance of cellular homeostasis. For example, after stress denaturation, Hsp70 and heat shock cognate (Hsc)70 contribute to the refolding of a broad range of substrate proteins (Young et al 2003). Furthermore, HSP70 proteins function with their cochaperones to prevent the aggregation of nonnative proteins through association with hydrophobic areas of substrate molecules (Mayer and Bukau 2005). Besides the functions that are linked to its intracellular location, the highly stress inducible member of the HSP70 family, Hsp72, has recently been detected in the peripheral circulation of healthy humans (Pockley et al 1998). The concentration of extracellular (e)Hsp72 increases significantly under stressful conditions such as exercise (Walsh et al 2001; Febbraio et al 2002; Lancaster et al 2004) and trauma (Pittet et al 2002). This finding has led to the proposal that eHsp72 release could have potent systemic roles. For example in vitro, eHsp72 is capable of inducing proinflammatory cytokine production from antigen-presenting cells (Asea et al 2000), as well as activating the complement cascade (Prohaszka et al 2002). These findings support suggestions that Hsp72 might act as a “danger signal” to the immune system (Matzinger 1998).
In the relatively few human studies to date that have measured eHsp72 in response to a variety of stressors, there is a disparity in the reported concentrations of Hsp72, which appears to be linked to the method in which the collected blood sample is treated. For example, while investigating the effect of exercise duration and intensity on eHsp72 concentration, Fehrenbach et al (2005) reported differences between plasma-derived and serum-derived Hsp72 despite similar exercise loads and subject characteristics between trials; for example, the assayed concentrations of plasma Hsp72 ranged from resting values of ∼1.5 ng/mL to 17 ng/mL immediately postexercise, whereas serum concentrations were much lower (∼0.1 ng/mL to maximum values of only 1.3 ng/mL). In addition, a review of human exercise studies obtaining eHsp72 measurements showed typical eHsp72 concentrations (rest–postexercise maximum) of 0.1–1.3 ng/mL in serum samples (Walsh et al 2001; Febbraio et al 2002, 2004; Lancaster et al 2004), compared with ranges of 1.5– 42 ng/mL for ethylenediaminetetraacetic acid (EDTA) plasma (Fehrenbach et al 2005; Peake et al 2005). Although it is acknowledged that the nature of the exercise in these studies might have an effect on the assayed concentrations, the subject characteristics and protocol used in the studies by Walsh et al (2001) and Fehrenbach et al (2005) were very similar, yet reported postexercise eHsp72 concentrations were markedly different (serum mean 1.02 ng/mL; and plasma mean 4.2 ng/mL, respectively). Furthermore, it is worth noting that all of these exercise studies used the same enzyme-linked immunosorbent assay to determine eHsp72 (EKS-700, Stressgen Biotechnologies, Victoria, BC, Canada). This difference between plasma and serum Hsp72 concentration clearly poses a problem when comparisons between studies are made to assess the degree to which certain independent variables affect the Hsp72 concentration.
A clear difference between plasma and serum is the formation and subsequent removal of the fibrin clot in serum. Because a key intracellular role of Hsp72 involves chaperoning aggregated proteins (Mayer and Bukau 2005), it is possible that eHsp72 binds to proteins involved in the clotting process. Accordingly, we hypothesized that assayed concentrations from serum-derived samples would be lower compared with plasma-derived blood samples. Therefore, our aim was to investigate the effect of blood handling on postexercise eHsp72 concentration with commonly used specimen tubes and blood handling procedures. Because Hsp72 is present in erythrocytes (Gromov and Celis 1991), it stands to reason that hemolysis as a consequence of venepuncture might alter the assayed Hsp72 concentration. Consequently, our secondary aim was to examine the relationship between eHsp72 and free hemoglobin in all assayed samples.
RESULTS
Effect of blood collection tube on postexercise eHsp72 concentration
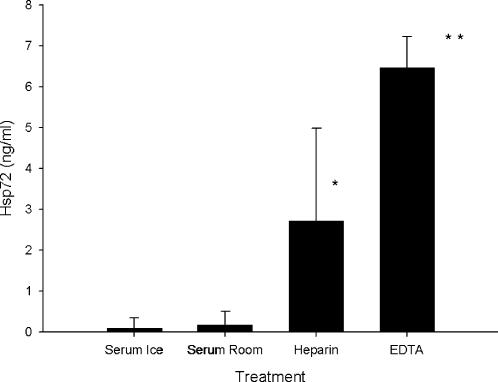
Analysis of variance revealed a main effect of blood collection tube on postexercise eHsp72 concentration (F(1.1,8.8) = 76.9, P < 0.001, η2 = 0.91) with a large effect size (Cohen 1988). Post hoc tests indicated that EDTA-treated blood had a significantly (P < 0.001) higher concentration of Hsp72 than all other treatments (Fig 1), whereas lithium heparin–treated blood had a significantly higher concentration of Hsp72 than both serum derived on ice (SI) and serum derived at room temperature (SR; P < 0.05 compared with both SI and SR), with no difference between SR and SI (Fig 1). eHsp72 was detected by enzyme-linked immunosorbent assay in only 1 serum ice sample, in 2 serum room samples, in 7 heparinized plasma samples, and in all 9 EDTA samples. The mean intra-assay CV of the 9 duplicate EDTA samples (4.8%) was lower than all other specimen types (SI, 7.0; SR, 19.5; LH, 17.4%).
Fig 1.
eHsp72 concentration in blood treated with serum (clotted on ice and at room temperature), heparin, and EDTA after exercise. Diluted (1:5) serum and plasma samples from 9 recreationally active men (mean ± SD; age 27.6 ± 0.9 years, height 178.6 ± 1.8 cm, body mass 75.4 ± 2.7 kg, estimated VO2max 54.3 ± 2.1 mL/kg/min) were assayed for eHsp72 in duplicate with a commercially available enzyme-linked immunosorbent assay (Stressgen Biotechnologies, Victoria, BC, Canada). The intra-assay coefficient of variation was calculated at 8.6%. Immediately on completion of the exercise time trial, participants were seated and blood samples were collected by venepuncture (22-gauge needle: Greiner Bio-one, Stonehouse, UK) from an antecubital vein into 4 different polypropylene specimen tubes in the following standardized draw order to prevent contamination: 2 × serum tubes, 1 × lithium heparin tube, and finally 1 × EDTA tube (Becton Dickenson Vacutainer, Oxford, UK). A tourniquet was used but was removed once blood flow had begun. Serum tubes were set on ice or at room temperature for 1 hour and were then centrifuged at 1500 × g at 4°C for 10 minutes before the supernatant was aspirated and frozen at −40°C for later analysis. Plasma tubes were handled according to the same centrifugation and freezing process. Differences in eHsp72 concentration between blood specimen tubes were analyzed with a 1-way repeated measures analysis of variance (ANOVA) and eta-squared effect size (η2) with greenhouse geisser adjustment where appropriate. Significant differences between methods following main effects were tested with Bonferroni post hoc comparisons. * P < 0.05 vs serum ice and serum room temperature, ** P ≤ 0.001 vs all other specimen tubes. Values are mean ± SD
Effect of hemolysis of eHsp72 concentration
To determine the extent of hemolysis in all assayed samples, free hemoglobin was assessed with a spectrophotometric scanning technique (Blakney and Dinwoodie 1975). Plasma samples were read at 562, 577, and 602 nm on a spectrophotometer (U2800 UV, Hitachi, Japan), and the resulting data were used in conjunction with the absorption coefficient of oxyhemoglobin to relate absorption to plasma hemoglobin concentration. Two samples showed clear visual evidence of extensive hemolysis and demonstrated very high concentrations of eHsp72 (14.2 and 194.2 ng/mL). These samples were withdrawn from all analyses. The correlation between free hemoglobin and eHsp72 in the remaining samples (with detectable eHsp72 values) was r = 0.36 (P = 0.133). Furthermore, there were no systematic trends in free hemoglobin between the collection methods.
DISCUSSION
In this study, we investigated the effects of different blood handling procedures on postexercise eHsp72 concentration in humans. In line with our hypothesis, eHsp72 assayed in plasma was significantly higher than that derived from serum. On review of previous studies employing different blood handling techniques, differences in eHsp72 concentration between serum and plasma can be expected (Walsh et al 2001; Febbraio et al 2002, 2004; Fehrenbach et al 2005). However, this is to our knowledge the first study to demonstrate a clear difference in eHsp72 concentration with the use of serum and plasma blood specimen tubes in a controlled within-subject design.
In serum, the bulk of fibrin and fibrinogen have been removed by the fibrillar clot, as well as platelets that have become enmeshed within the clot (Lundblad 2005). Because the fluid compartment remains in contact with the blood cells for a longer period of time compared with plasma, the likelihood of artifactual changes in serum could be greater (Hrubec et al 2002). Indeed, differences in assayed concentrations of proteins such as albumin, creatine kinase, and total protein between serum and plasma have been reported (Ladenson et al 1974). Because of the intracellular roles of Hsp72 in chaperoning aggregated proteins, it is possible that eHsp72 could bind to proteins involved in the clotting process, such as fibrin or fibrinogen, thus decreasing the assayed concentration of eHsp72. Indeed, Hsp72 peptide complexes have been shown to bind to the CD91 receptor on antigen-presenting cells (Basu et al 2001), and other high–molecular mass HSPs (GP96) have recently been shown to bind to CD91 on human platelets (Hilf et al 2002). Furthermore, low– molecular mass HSPs (Hsp20) have been shown to bind specifically to platelets after endothelial injury in hamsters (Kozawa et al 2002). Therefore, it could be that platelets might also be a potential target for eHsp72 during clotting. The fibrillar clot is an important aspect of the innate immune system because of its ability to entrap invading bacteria and prevent its spread throughout the body (Dunn and Simmons 1982). In the horseshoe crab, a number of immune effector proteins are known to bind to the fibrils of formed blood clots, and it has been suggested that rather than just a passive entrapment device, the blood clot is a delivery mechanism for proteins and cells that are capable of inducing an immune response (Armstrong and Armstrong 2003). Indeed, through the αMβ2/Mac-1 integrin, fibrin(ogen) might attract adhesion of neutrophils and macrophages and subsequent innate immune resources in rodents (Flick et al 2004). Although further research is required to confirm these functions in humans, the immunostimulatory role of Hsp72 places this as a further possible explanation for Hsp72 binding to the fibrillar clot.
Aside from the clear differences in eHsp72 concentration in serum and plasma, the current data also suggest that plasma eHsp72 concentrations are dependent on the anticoagulant used. EDTA plasma provided 2.5-fold higher eHsp72 concentrations over lithium heparin. Lithium heparin activates anti-thrombin, which prevents thrombin from converting fibrinogen to fibrin. However, EDTA is a metal ion chelator, and in the case of whole blood, it binds with calcium, which is crucial in thrombin formation and thus blood clotting. Substrate protein binding of Hsp72 is adenosine triphosphate (ATP) dependent (Mayer and Bukau 2005), and because magnesium forms part of the ATPase domain of Hsp72 (Mayer and Bukau 2005), it is possible that EDTA binds with Mg2+. Hsp72 might therefore be prevented from binding to substrate proteins involved in the clotting process. In current work in our laboratory, we are investigating whether eHsp72 in vitro does indeed bind to such clotting proteins. Although it could be argued that the use of anticoagulant chemicals such as heparin and EDTA might interfere with analyte detection, the small concentration of EDTA within a single vacutainer was unlikely to inhibit the detection of eHsp72 concentration in this study, particularly considering the magnitude of the effect. A further advantage of EDTA is the increased confidence in the precision of derived concentrations, as demonstrated by the lower coefficient of variation between the duplicate samples compared with the other blood collection tubes.
One of the problems with serum-derived Hsp72 is that concentrations are typically at or below the detectable range of the assay (standard curve 0.78–50 ng/mL, sensitivity 0.5 ng/mL). This therefore increases the chance of deriving undetectable samples or relying on extrapolated data from the standard curve. Furthermore, if Hsp72 does indeed bind to the blood clot, this raises questions as to whether the extracellular concentration reported in studies with serum accurately reflects the concentrations of circulating Hsp72 in vivo. Although this should not detract from studies reporting changes in Hsp72 concentration in response to a certain stressor, it does cause problems in cross-sectional studies, in particular, when direct comparisons are made with the Hsp72 concentration of other fluids or when correlations are performed with certain variables of interest. Furthermore, because it is at present unclear as to the source of eHsp72 during exercise, release from organs or cells could be wrongly discounted if Hsp72 is largely undetectable in serum. These points stress the need for stringent control procedures when serum is used because it stands to reason that changes in clotting time and temperature in particular are likely to affect the assayed concentration of Hsp72. Furthermore, the use of a glass or plastic container can markedly alter the quality of blood clotting and subsequent serum (Lundblad 2005).
Because Hsp72 is present in erythrocytes (Gromov and Celis 1991) and hemolysis is associated with necrosis and membrane damage to these cells, it is possible that hemolysis could affect the assayed eHsp72 concentration. Indeed, 2 samples clearly showing visual evidence of hemolysis produced very high concentrations of eHsp72 (14.2 and 194.2 ng/mL) and free hemoglobin (2.43 and 22.8 g/L). Inclusion of these data in the statistical analyses provided a very high correlation between eHsp72 concentration and hemolysis. However, when removed as outliers, no significant correlation appeared between eHsp72 concentration and free hemoglobin. Therefore, our data suggests that although extensive hemolysis because of poor venepuncture could elevate eHsp72 concentration, insufficient evidence is present now to suggest that hemolysis as a consequence of this type of exercise might affect eHsp72 concentration.
The results demonstrate that blood treated with an anticoagulant provides significantly higher concentrations of Hsp72 than serum-derived Hsp72, which is more likely to represent circulating in vivo concentrations. Because previous research has tended to report serum data at the lowest point of the detectable range of the assay, it is recommended that EDTA-coated specimen tubes be used in future investigations into the in vivo Hsp72 stress response. That eHsp72 binds to the fibrillar clot in vivo is an interesting hypothesis that warrants further investigation.
Table 1.
Mean (± SD) time, heart rate, power output, pre-exercise and final rectal temperature during time trial exercise in nine recreationally active males. Mean and standard deviation values for serum and plasma were obtained from previous literature (Walsh et al 2001; Fehrenbach et al 2005) and implemented into a freely available internet power calculator (http://calculators.stat.ucla.edu/powercalc/). This provided a recommended n of 6.8 (α=0.01, β=0.9). A total of nine subjects recruited was therefore deemed to provide sufficient statistical power. All participants gave written informed consent. The entire exercise protocol consisted of a maximal incremental test to volitional exhaustion on a cycle ergometer (Lode Excalibur, Gronigen, The Netherlands) to determine peak power output (288.2 ± 13.7W) followed by an individually specific time-trial (total work 415.2 ± 19.7 kJ (Jeukendrup et al 1996) in hot environmental conditions (34.6 ± 0.1 °C, 42.4 ± 1.9 % relative humidity). Heart rate and rectal temperature were monitored by telemetry (Polar electro Oy, Finland) and digital thermister probe (YSI Precision, Ohio) respectively. A one way repeated measures ANOVA with tukey's post hoc comparisons revealed that performing the time-trial in a hot environment was successful in raising rectal temperature (F(10,80) = 147.9, P < 0.001) which had increased significantly after 20% of the time-trial completed, with a total increas of 1.2°C above pre-exercise temperature. Water was allowed throughout the exercise protocol ad libidum

Acknowledgments
The authors thank Dr Hans-Peter Kubis for technical advice and discussion of the concepts addressed in this paper.
REFERENCES
- Armstrong PB, Armstrong MT. The decorated clot: binding of agents of the innate immune system to the fibrils of the limulus blood clot. Biol Bull. 2003;205:201–203. doi: 10.2307/1543252.0006-3185(2003)205[0201:TDCBOA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697.1078-8956(2000)006[0435:HSCPTA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x.1074-7613(2001)014[0303:CIACRF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Blakney GB, Dinwoodie AJ. A spectrophotometric scanning technique for the rapid determination of plasma hemoglobin. Clin Biochem. 1975;8:96–102. doi: 10.1016/s0009-9120(75)91005-x.0009-9120(1975)008[0096:ASSTFT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cohen J 1988 Statistical Power Analysis for the Behavioural Sciences. Lawrence Erlbaum Associates, Mahwah, NJ. [Google Scholar]
- Dunn DL, Simmons RL. Fibrin in peritonitis. III. The mechanism of bacterial trapping by polymerizing fibrin. Surgery. 1982;92:513–519.0039-6060(1982)092[0513:FIPITM]2.0.CO;2 [PubMed] [Google Scholar]
- Febbraio MA, Mesa JL, and Chung J. et al. 2004 Glucose ingestion attenuates the exercise-induced increase in circulating heat shock protein 72 and heat shock protein 60 in humans. Cell Stress Chaperones. 9:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol (Lond) 2002;544:957–962. doi: 10.1113/jphysiol.2002.025148.0022-3751(2002)544[0957:EIHROH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Voelker K, Northoff H, Mooren FC. Exercise intensity and duration affect blood soluble HSP72. Int J Sports Med. 2005;26:552–557. doi: 10.1055/s-2004-830334.0172-4622(2005)026[0552:EIADAB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741.0021-9738(2004)113[1596:LEOFVT]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromov PS, Celis JE. Identification of two molecular chaperons (HSX70, HSC70) in mature human erythrocytes. Exp Cell Res. 1991;195:556–559. doi: 10.1016/0014-4827(91)90412-n.0014-4827(1991)195[0556:IOTMCH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hilf N, Singh-Jasuja H, Schwarzmaier P, Gouttefangeas C, Rammensee HG, Schild H. Human platelets express heat shock protein receptors and regulate dendritic cell maturation. Blood. 2002;99:3676–3682. doi: 10.1182/blood.v99.10.3676.0006-4971(2002)099[3676:HPEHSP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hrubec TC, Whichard JM, Larsen CT, Pierson FW. Plasma versus serum: specific differences in biochemical analyte values. J Avian Med Surg. 2002;16:101–105.1082-6742(2002)016[0101:PVSSDI]2.0.CO;2 [Google Scholar]
- Jeukendrup A, Saris WH, Brouns F, Kester AD. A new validated endurance performance test. Med Sci Sports Exerc. 1996;28:266–270. doi: 10.1097/00005768-199602000-00017.0195-9131(1996)028[0266:ANVEPT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kozawa O, Matsuno H, Niwa M, Hatakeyama D, Oiso Y, Kato K, Uematsu T. HSP20, low–molecular-weight heat shock–related protein, acts extracellularly as a regulator of platelet functions: a novel defense mechanism. Life Sci. 2002;72:113–124. doi: 10.1016/s0024-3205(02)02144-6.0024-3205(2002)072[0113:HLHSPA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ladenson JH, Tsai LM, Michael JM, Kessler G, Joist JH. Serum versus heparinized plasma for eighteen common chemistry tests: is serum the appropriate specimen? Am J Clin Pathol. 1974;62:545–552. doi: 10.1093/ajcp/62.4.545.0002-9173(1974)062[0545:SVHPFE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lancaster GI, Moller K, Nielsen B, Secher NH, Febbraio MA, Nybo L. Exercise induces the release of heat shock protein 72 from the human brain in vivo. Cell Stress Chaperones. 2004;9:276–280. doi: 10.1379/CSC-18R.1.1466-1268(2004)009[0276:EITROH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad RL. Considerations for the use of blood plasma and serum for proteomic analysis. Int J Gastroenterol. 2005;1:1–11.1528-8323(2005)001[0001:CFTUOB]2.0.CO;2 [Google Scholar]
- Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143.1044-5323(1998)010[0399:AISOD]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6.1420-682X(2005)062[0670:HCCFAM]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake JM, Suzuki K, Hordern M, Wilson G, Nosaka K, Coombes JS. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur J Appl Physiol. 2005;95:514–521. doi: 10.1007/s00421-005-0035-2.0301-5548(2005)095[0514:PCCIRT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma. 2002;52:611–617. doi: 10.1097/00005373-200204000-00001.0022-5282(2002)052[0611:SLOHME]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–377. doi: 10.3109/08820139809022710.0882-0139(1998)027[0367:DOHSPH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Prohaszka Z, Singh M, Nagy K, Kiss E, Lakos G, Duba J, Fust G. Heat shock protein 70 is a potent activator of the human complement system. Cell Stress Chaperones. 2002;7:17–22. doi: 10.1379/1466-1268(2002)007<0017:hspiap>2.0.co;2.1466-1268(2002)007[0017:HSPIAP]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:eishih>2.0.co;2.1466-1268(2001)006[0386:EISHIH]2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Barral JM, Ulrich HF. More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci. 2003;28:541–547. doi: 10.1016/j.tibs.2003.08.009.0376-5067(2003)028[0541:MTFLFO]2.0.CO;2 [DOI] [PubMed] [Google Scholar]