Abstract
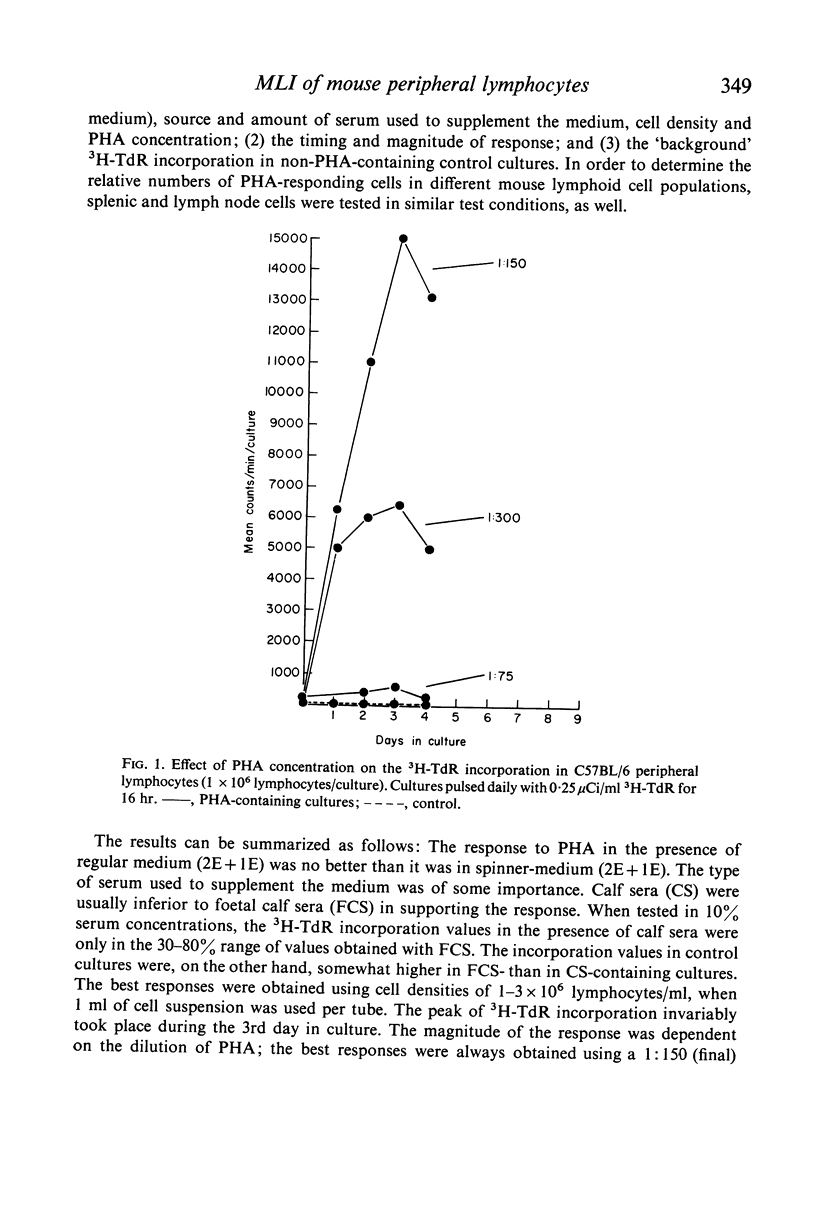
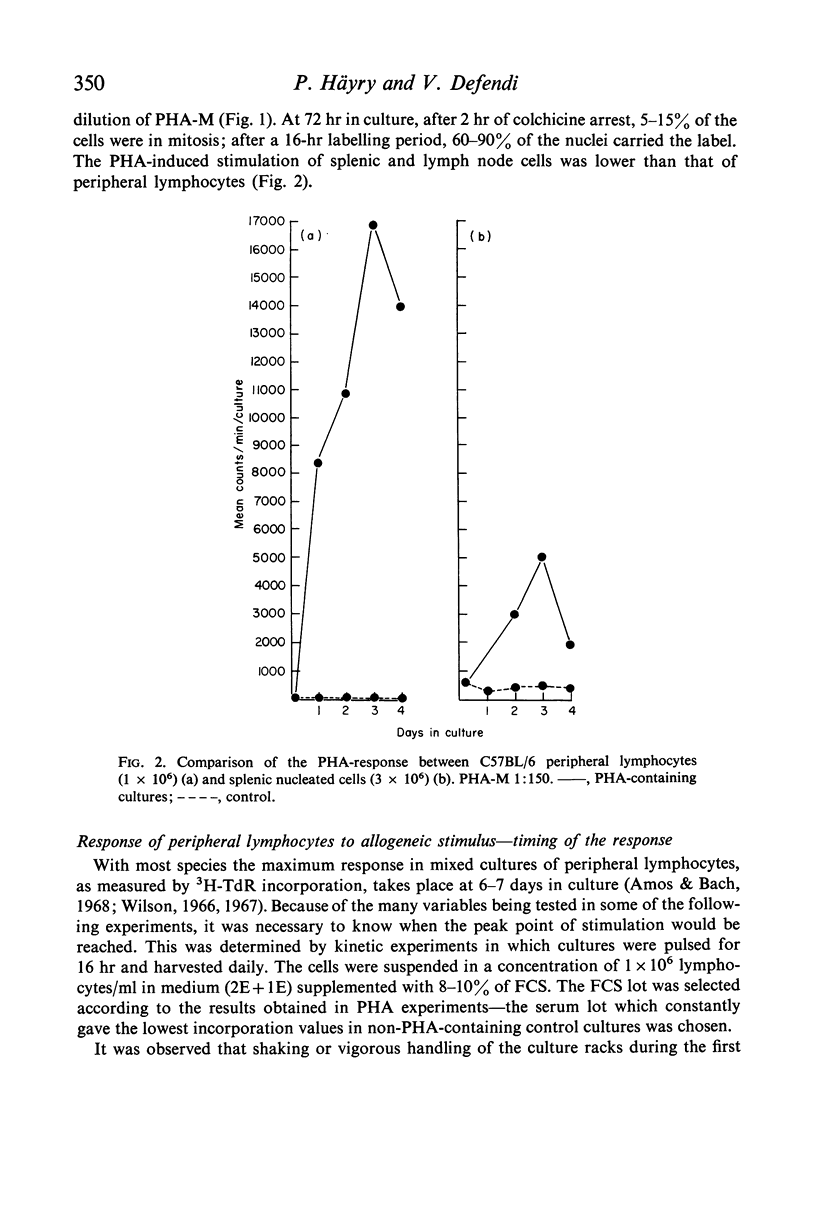
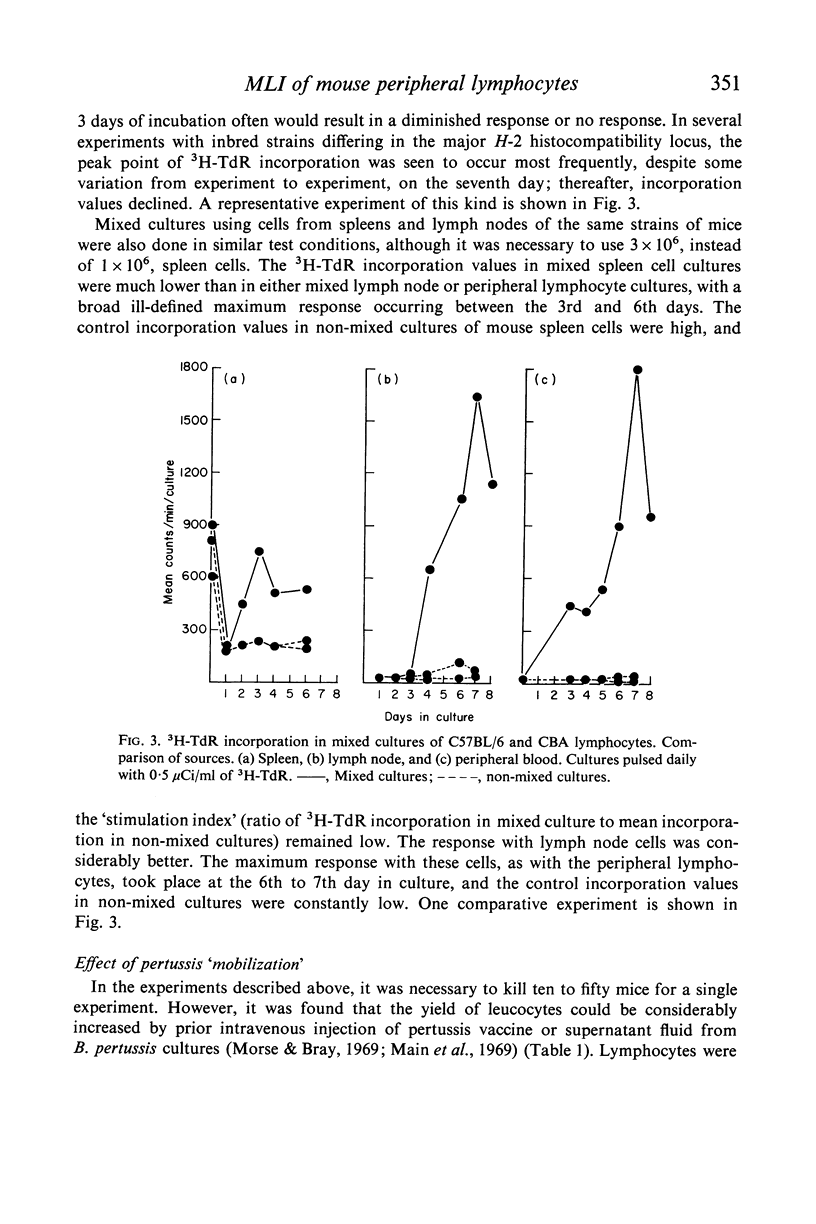
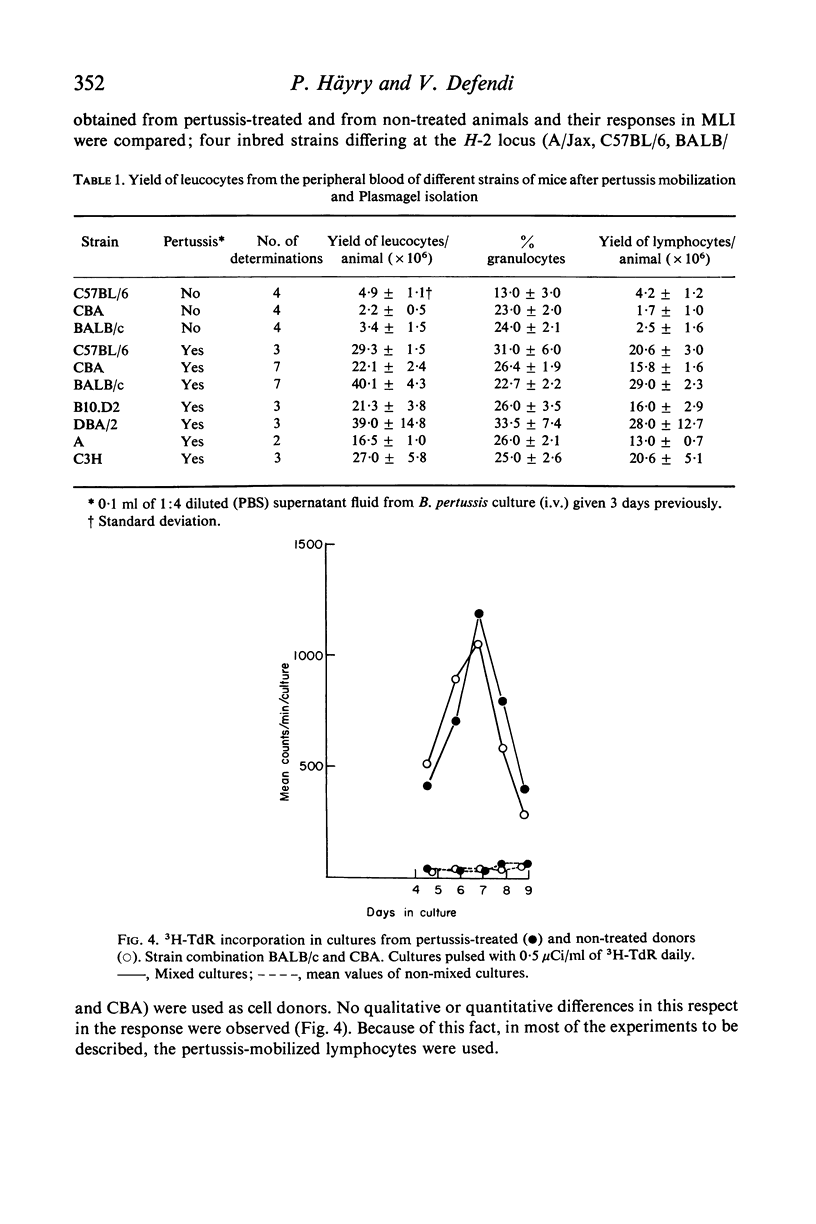
We have adapted mouse peripheral lymphocytes to culture as a preliminary step in designing a model for the study of allograft immunity in vitro. The isolation of peripheral leucocytes is facilitated by using Plasmagel® as an erythrocyte-agglutinating agent. The yield of leucocytes can be considerably increased by intravenous injection of the donor animals with supernatant fluid from Bordetella pertussis cultures and the lymphocytes thus mobilized react both to phytohaemagglutinin (PHA) and allogeneic stimulus, as do lymphocytes from untreated animals. Preparations which contain more than 25–50 RBC/WBC are refractory in the mixed lymphocyte interaction (MLI). The optimum cell density for the proliferative response is approximately 1–3 × 106 lymphocytes/ml. Various nutritive milieu were tested and found to have little influence on the MLI; both normal and suspension media behaved in a similar manner. PHA causes a vigorous proliferative response in mouse peripheral lymphocytes, the 3H–TdR incorporation values in PHA-containing cultures at peak point of stimulation (3rd day) being up to 1000 times those observed for control cultures. The allogeneic response in the MLI takes place later (6th to 7th day) and is weaker, about one-tenth the PHA response, when strains differing at the H-2 locus are used as cell donors. Because the specific proliferative response to allogeneic stimulus in mixed culture, regardless of the way it is measured, is indistinguishable from the response produced by other non-specific factors, these other factors must be critically excluded. It appears that supplementing the culture medium with low concentrations of certain lots of foetal calf or agamma-newborn-calf serum permits the study of the specific response at an optimum sensitivity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos D. B., Bach F. H. Phenotypic expressions of the major histocompatibility locus in man (HL-A): leukocyte antigens and mixed leukocyte culture reactivity. J Exp Med. 1968 Oct 1;128(4):623–637. doi: 10.1084/jem.128.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAIN B., VAS M. R., LOWENSTEIN L. THE DEVELOPMENT OF LARGE IMMATURE MONONUCLEAR CELLS IN MIXED LEUKOCYTE CULTURES. Blood. 1964 Jan;23:108–116. [PubMed] [Google Scholar]
- Bach F. H., Bock H., Graupner K., Day E., Klostermann H. Cell kinetic studies in mixed leukocyte cultures: an in vitro model of homograft reactivity. Proc Natl Acad Sci U S A. 1969 Feb;62(2):377–384. doi: 10.1073/pnas.62.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Further studies of the stimulation of DNA synthesis in cultures of spleen cell suspensions by homologous cells in inbred strains of mice and rats. J Exp Med. 1965 Oct 1;122(4):759–770. doi: 10.1084/jem.122.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- HILDEMANN W. H. IMMUNOLOGICAL PROPERTIES OF SMALL BLOOD LYMPHOCYTES IN THE GRAFT-VERSUS-HOST REACTION IN MICE. Transplantation. 1964 Jan;2:38–47. doi: 10.1097/00007890-196401000-00005. [DOI] [PubMed] [Google Scholar]
- HIRSCHHORN K., BACH F., RAPAPORT F. T., CONVERSE J. M., LAWRENCE H. S. THE RELATIONSHIP OF IN VITRO LYMPHOCYTE COMPATIBILITY TO HOMOGRAFT SENSITIVITY IN MAN. Ann N Y Acad Sci. 1964 Nov 30;120:303–306. doi: 10.1111/j.1749-6632.1964.tb34729.x. [DOI] [PubMed] [Google Scholar]
- MOORHEAD P. S., NOWELL P. C., MELLMAN W. J., BATTIPS D. M., HUNGERFORD D. A. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960 Sep;20:613–616. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- Morse S. I., Bray K. K. The occurrence and properties of leukocytosis and lymphocytosis-stimulating material in the supernatant fluids of Bordetella pertussis cultures. J Exp Med. 1969 Mar 1;129(3):523–550. doi: 10.1084/jem.129.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M. R. The mixed lymphocyte reaction: an in vitro test for tolerance. J Exp Med. 1968 May 1;127(5):879–890. doi: 10.1084/jem.127.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staats J. Standardized nomenclature for inbred strains of mice: fourth listing. Cancer Res. 1968 Mar;28(3):391–420. [PubMed] [Google Scholar]
- Strander H., Cantell K. Production of interferon by human leukocytes in vitro. Ann Med Exp Biol Fenn. 1966;44(2):265–273. [PubMed] [Google Scholar]
- Wilson D. B., Blyth JL NOWELL P. C. Quantitative studies on the mixed lymphocyte interaction in rats. 3. Kinetics of the response. J Exp Med. 1968 Nov 1;128(5):1157–1181. doi: 10.1084/jem.128.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B. Quantitative studies on the mixed lymphocyte interaction in rats. I. Conditions and parameters of response. J Exp Med. 1967 Oct 1;126(4):625–654. doi: 10.1084/jem.126.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Silvers W. K., Nowell P. C. Quantitative studies on the mixed lymphocyte interaction in rats. II. Relationship of the proliferative response to the immunologic status of the donors. J Exp Med. 1967 Oct 1;126(4):655–665. doi: 10.1084/jem.126.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]


