Abstract
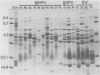
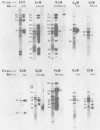
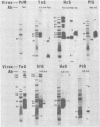
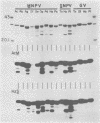
Immunological comparisons were made of baculovirus structural proteins by using a modification of the radioimmunological techniques described by Renart et al. (Proc. Natl. Acad. Sci. U.S.A. 76: 3116-3120, 1979) and Towbin et al. (Proc. Natl. Acad. Sci. U.S.A. 76: 4350-4354, 1979). Viral proteins were electrophoresed in polyacrylamide gels, transferred to nitrocellulose, and incubated with viral antisera, and the antibodies were detected with 125I-labeled Staphylococcus aureus protein A. Antisera were prepared to purified and intact virions from five baculoviruses: Autographa californica, Porthetria dispar, Trichoplusia ni, and Heliothis zea nuclear polyhedrosis viruses (NPVs) and T. ni granulosis virus (GV). These antisera were tested against the virion structural polypeptides of 17 different species of baculoviruses. Specific multiple-nucleocapsid NPV (MNPV), single-nucleocapsid NPV (SNPV), and GV virion polypeptides were shown to have similar antigenic determinants and thus be immunologically related. The molecular weights of the virion polypeptides with cross-reacting antigenic determinants were identified. Antisera prepared to purified A. californica and H. zea MNPV polyhedrin (the occlusion body protein from NPVs) recognized antigenic determinants on all the polyhedrins and granulins (occlusion body protein from GVs) that were tested. No immunological relationship was detected between A. californica MNPV polyhedrin and any of the A. californica MNPV virion structural polypeptides present on either the virus isolated from occlusion bodies or A. californica MNPV extracellular virus from infected-cell cultures.
Full text
PDF

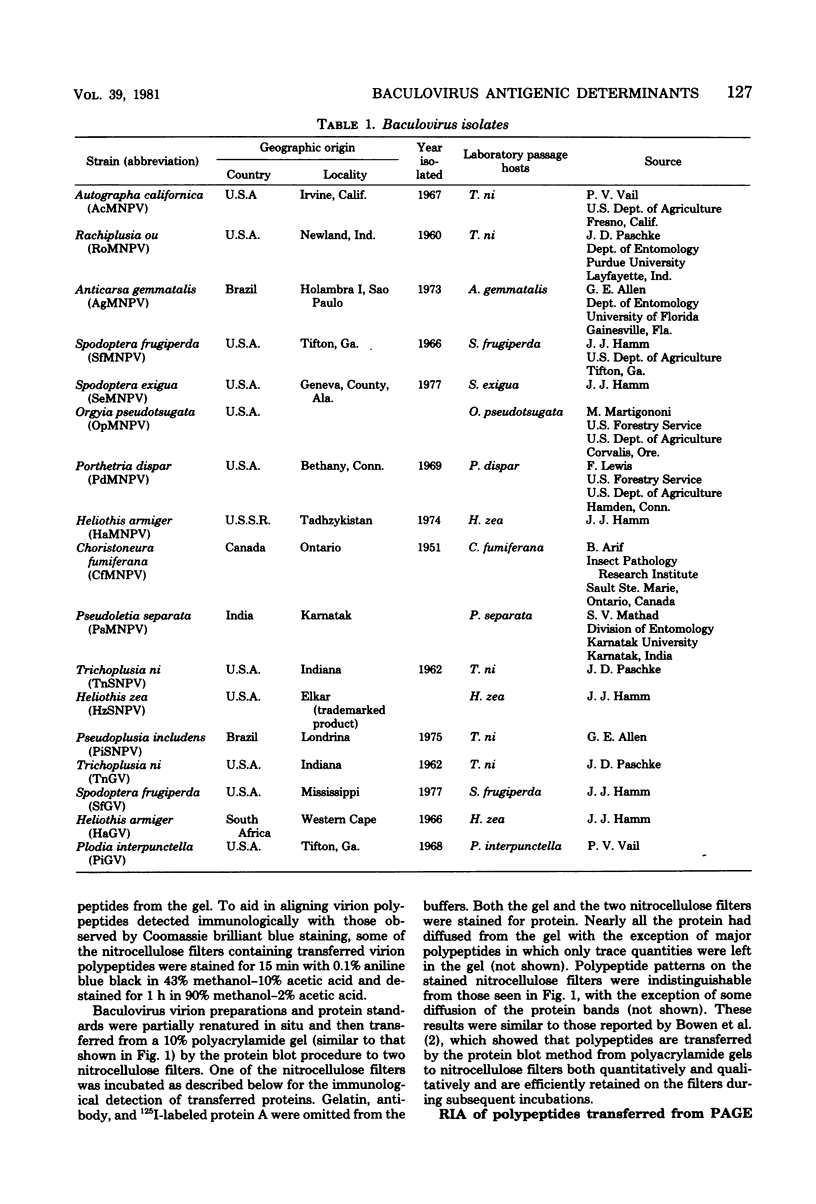

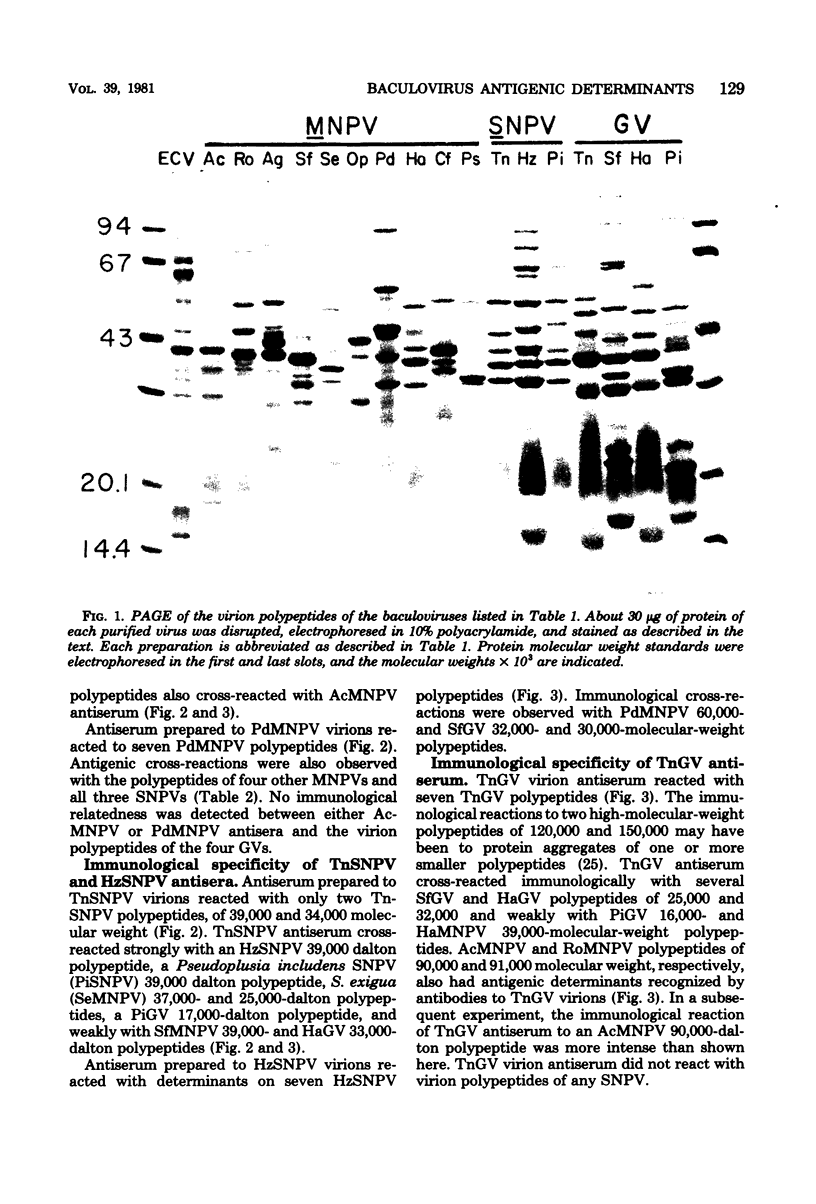
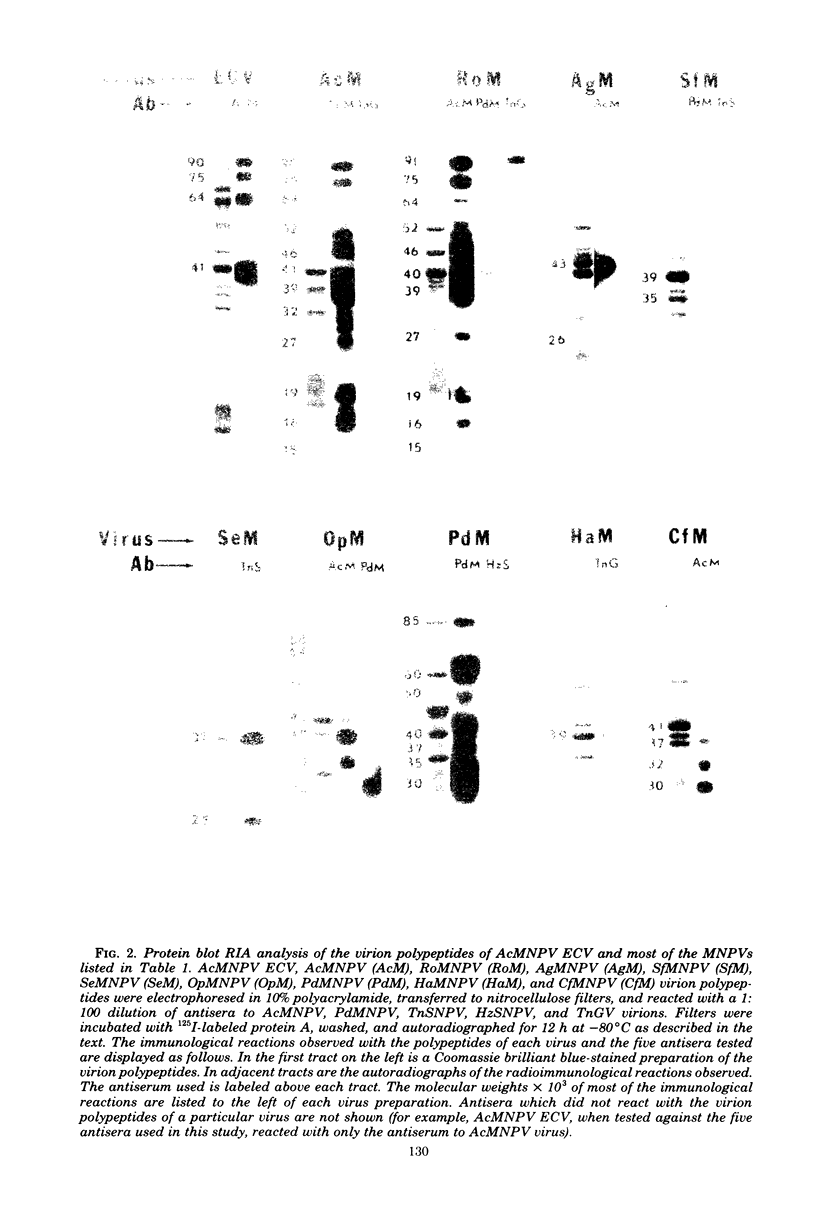

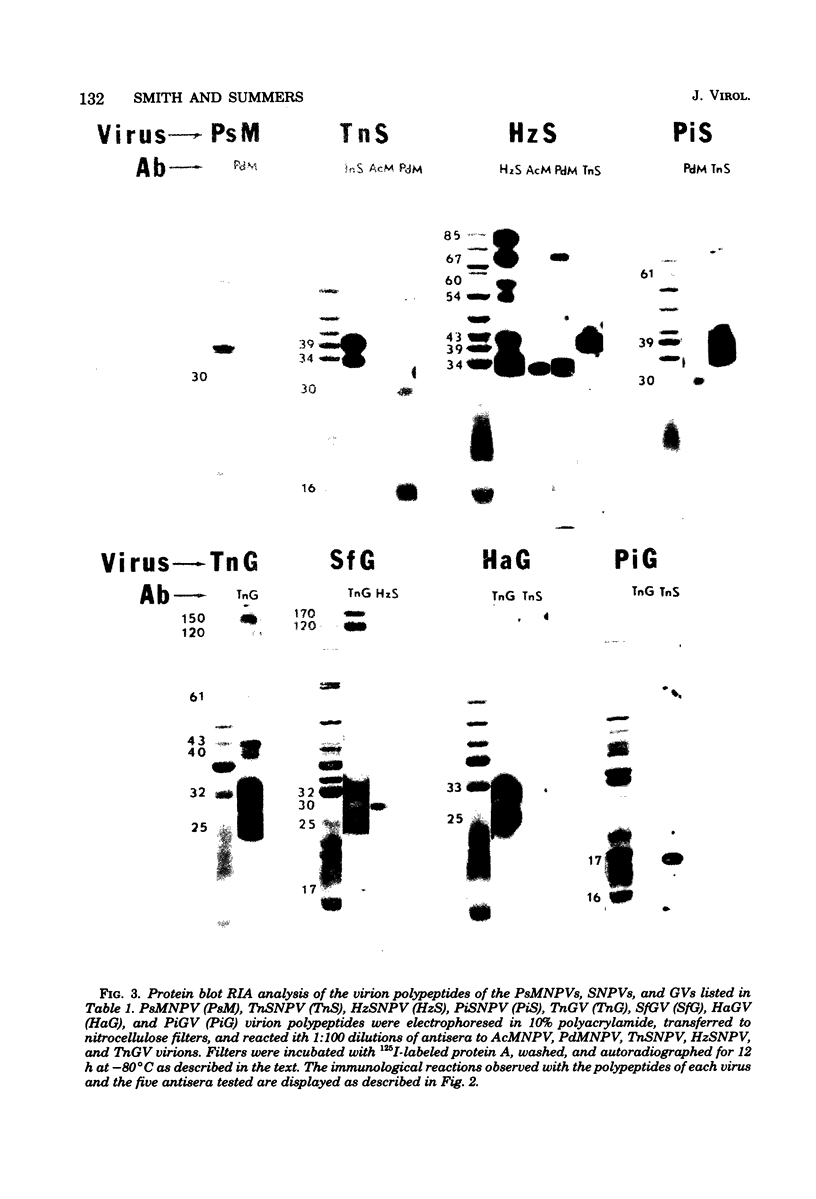
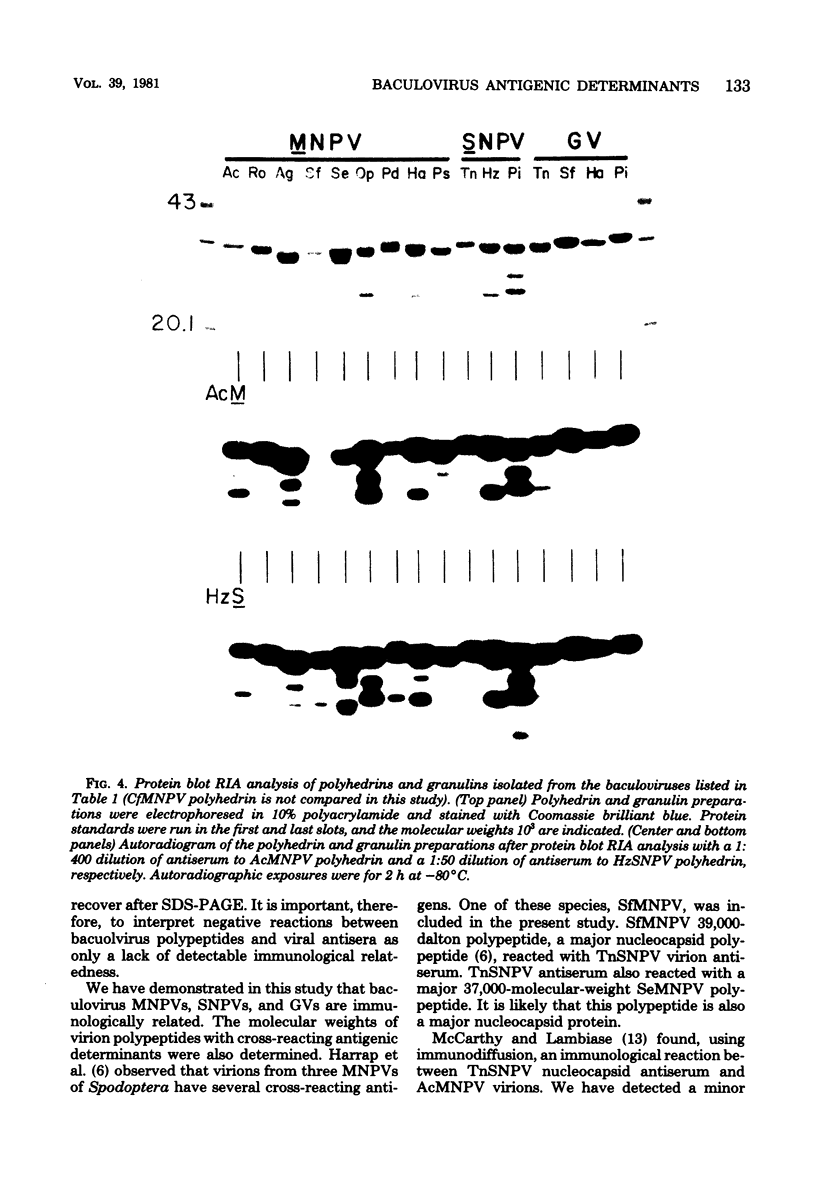




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Daniel M. D., Aaronson S. A. Immunological relationships of OMC-1, an endogenous virus of owl monkeys, with mammalian and avian type C viruses. J Virol. 1980 Jan;33(1):561–566. doi: 10.1128/jvi.33.1.561-566.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens E. B., Tjia S. T., Doerfler W. Infection of Spodoptera frugiperda cells with Autographa californica nuclear polyhedrosis virus I. Synthesis of intracellular proteins after virus infection. Virology. 1979 Dec;99(2):386–398. doi: 10.1016/0042-6822(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E., Tronick S. R., Aaronson S. A. Immunological relationships of an endogenous guinea pig retrovirus with prototype mammalian type B and type D retroviruses. J Virol. 1980 Jan;33(1):522–530. doi: 10.1128/jvi.33.1.522-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrap K. A., Payne C. C., Robertson J. S. The properties of three baculoviruses from closely related hosts. Virology. 1977 Jun 1;79(1):14–31. doi: 10.1016/0042-6822(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Kozlov E. A., Sidorova N. M., Serebryani S. B. Proteolytic cleavage of polyhedral protein during dissolution of inclusion bodies of the nuclear polyhedrosis viruses of Bombyx mori and Galleria mellonella under alkaline conditions. J Invertebr Pathol. 1975 Jan;25(1):97–101. doi: 10.1016/0022-2011(75)90288-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marier R., Jansen M., Andriole V. T. A new method for measuring antibody using radiolabeled protein A1 in a solid-phase radioimmunoassay. J Immunol Methods. 1979;28(1-2):41–49. doi: 10.1016/0022-1759(79)90326-0. [DOI] [PubMed] [Google Scholar]
- Renart J., Reiser J., Stark G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann G. F., Bailey T. J., Brimhall B., Becker R. R., Beaudreau G. S. Tryptic peptide analysis and NH2-terminal amino acid sequences of polyhedrins of two baculoviruses from Orgyia pseudotsugata. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4976–4980. doi: 10.1073/pnas.76.10.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. Restriction Map of Rachiplusia ou and Rachiplusia ou-Autographa californica Baculovirus Recombinants. J Virol. 1980 Jan;33(1):311–319. doi: 10.1128/jvi.33.1.311-319.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Expression of endogenous RNA C-type virus group-specific antigens in mammalian cells. J Virol. 1973 Sep;12(3):564–569. doi: 10.1128/jvi.12.3.564-569.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of mammalian RNA tumor viruses: relatedness of the interspecies antigenic determinants of the major internal protein. J Virol. 1975 Jun;15(6):1332–1341. doi: 10.1128/jvi.15.6.1332-1341.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. D., Egawa K. Physical and chemical properties of Trichoplusia ni granulosis virus granulin. J Virol. 1973 Nov;12(5):1092–1103. doi: 10.1128/jvi.12.5.1092-1103.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. D., Smith G. E. Baculovirus structural polypeptides. Virology. 1978 Feb;84(2):390–402. doi: 10.1016/0042-6822(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Smith G. E. Comparative studies of baculovirus granulins and polyhedrins. Intervirology. 1975;6(3):168–180. doi: 10.1159/000149469. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Smith G. E., Knell J. D., Burand J. P. Physical Maps of Autographa californica and Rachiplusia ou Nuclear Polyhedrosis Virus Recombinants. J Virol. 1980 Jun;34(3):693–703. doi: 10.1128/jvi.34.3.693-703.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweeten K. A., Bulla L. A., Consigli R. A. Characterization of an extremely basic protein derived from granulosis virus nucleocapsids. J Virol. 1980 Feb;33(2):866–876. doi: 10.1128/jvi.33.2.866-876.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman L. E., Summers M. D., Hsieh C. H. Occluded and nonoccluded nuclear polyhedrosis virus grown in Trichoplusia ni: comparative neutralization comparative infectivity, and in vitro growth studies. J Virol. 1976 Sep;19(3):820–832. doi: 10.1128/jvi.19.3.820-832.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman L. E., Summers M. D. Nuclear polyhedrosis virus detection: relative capabilities of clones developed from Trichoplusia ni ovarian cell line TN-368 to serve as indicator cells in a plaque assay. J Virol. 1975 Dec;16(6):1630–1637. doi: 10.1128/jvi.16.6.1630-1637.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilson A. C., Carlson S. S., White T. J. Biochemical evolution. Annu Rev Biochem. 1977;46:573–639. doi: 10.1146/annurev.bi.46.070177.003041. [DOI] [PubMed] [Google Scholar]