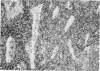Abstract
This paper describes the course of infection and development of immunity in guinea-pigs after intradermal inoculation of Leishmania enriettii, and the use of in vivo and in vitro techniques to characterize the immunological response to infection and artificial immunization.
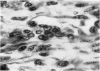
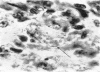
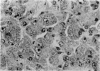
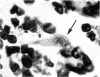
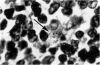
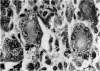
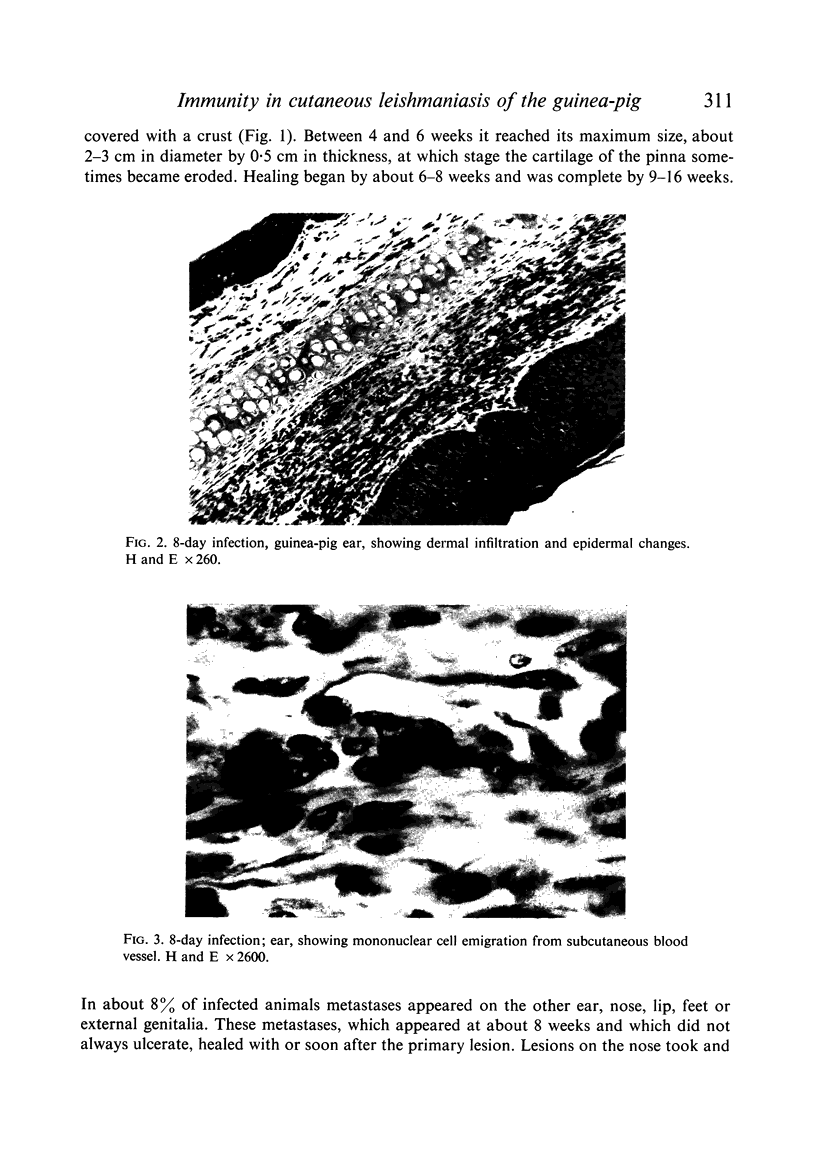
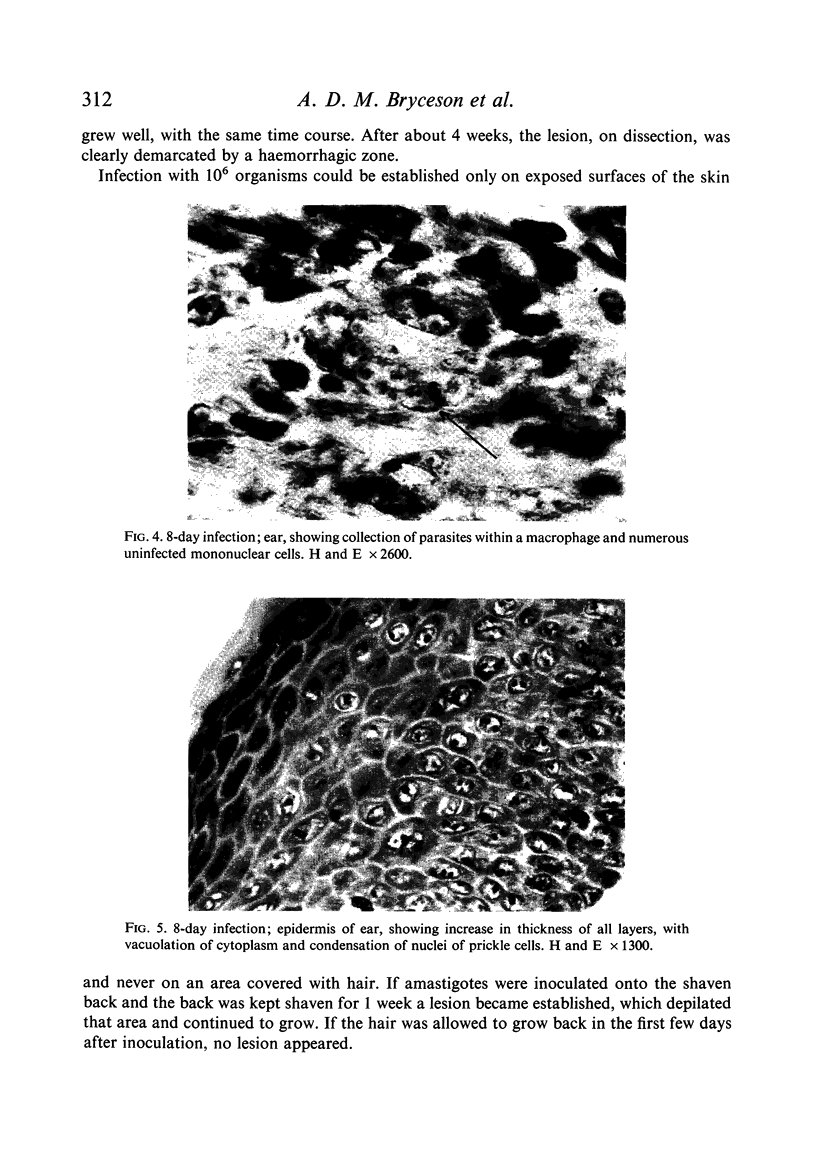
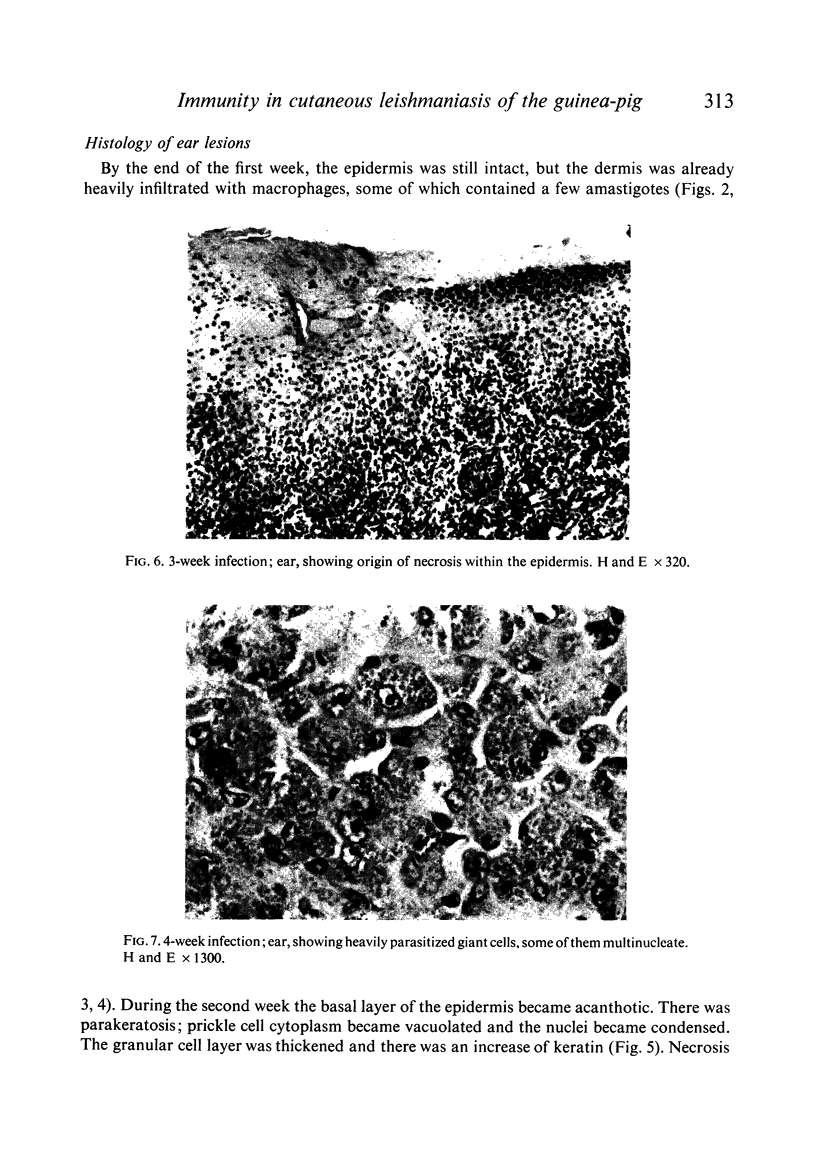
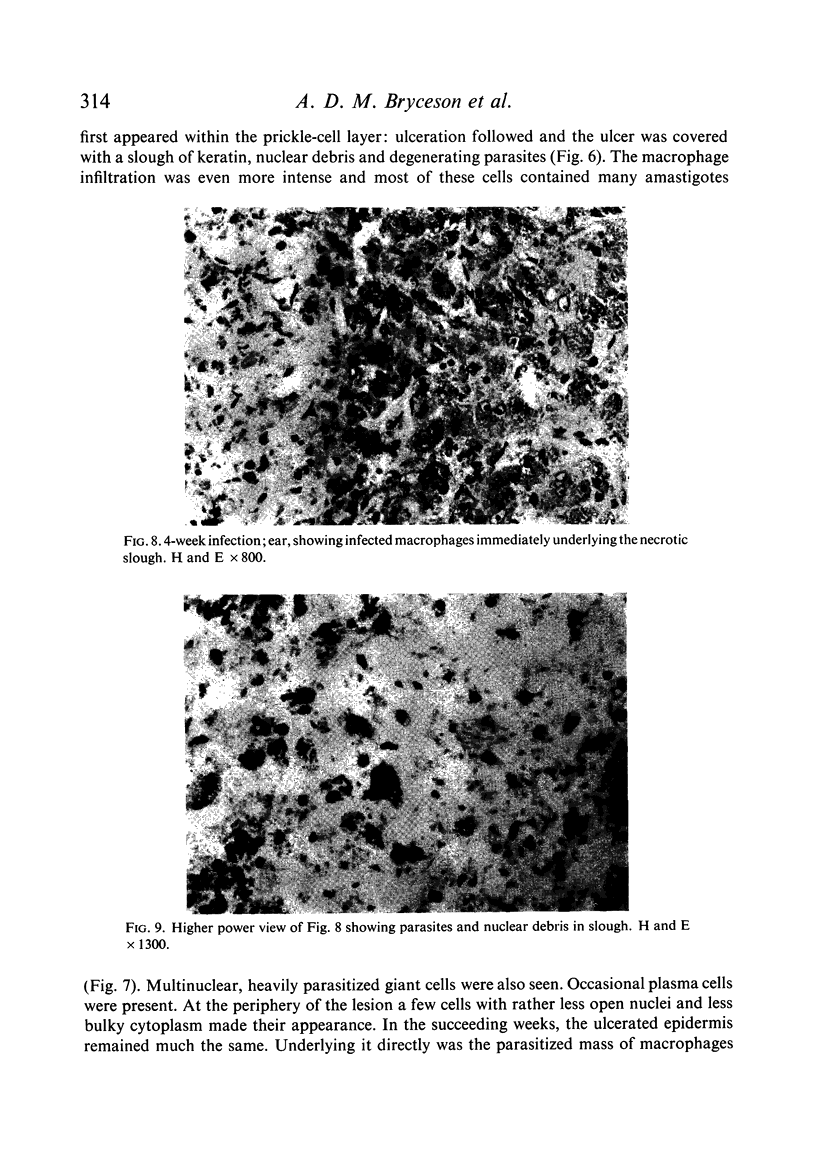
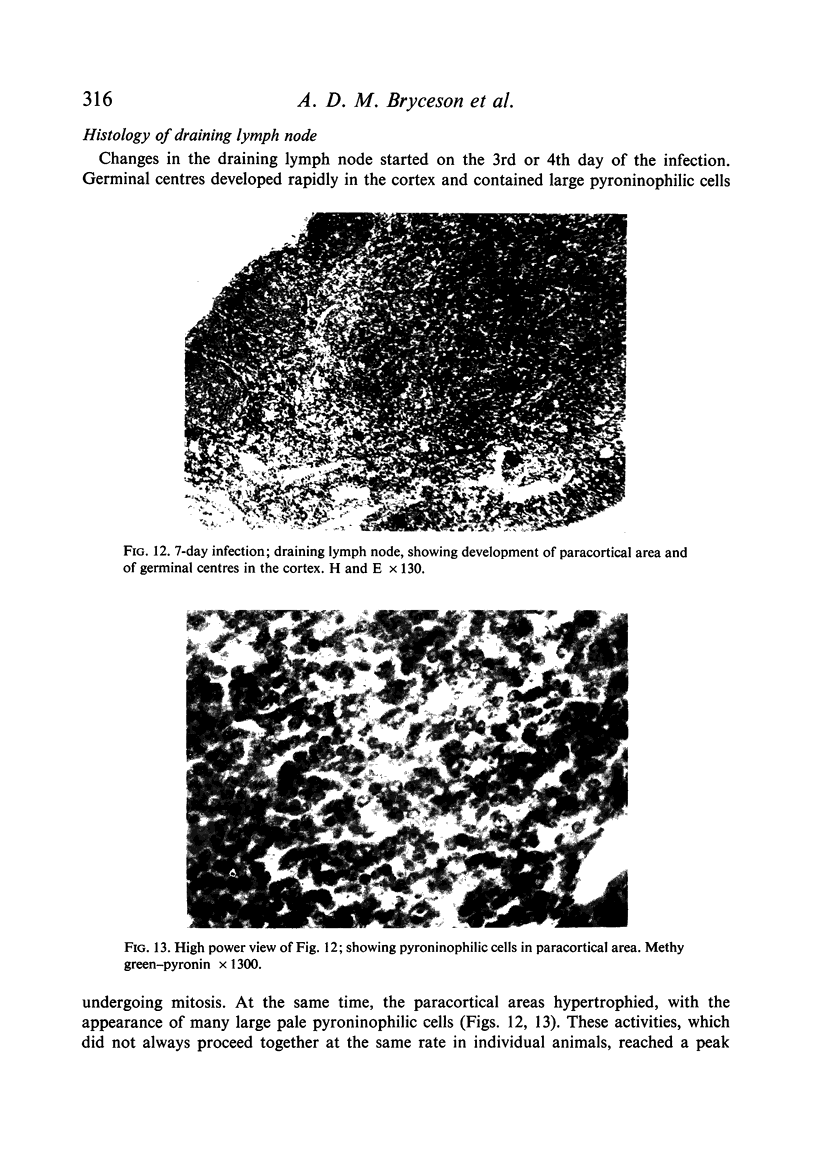
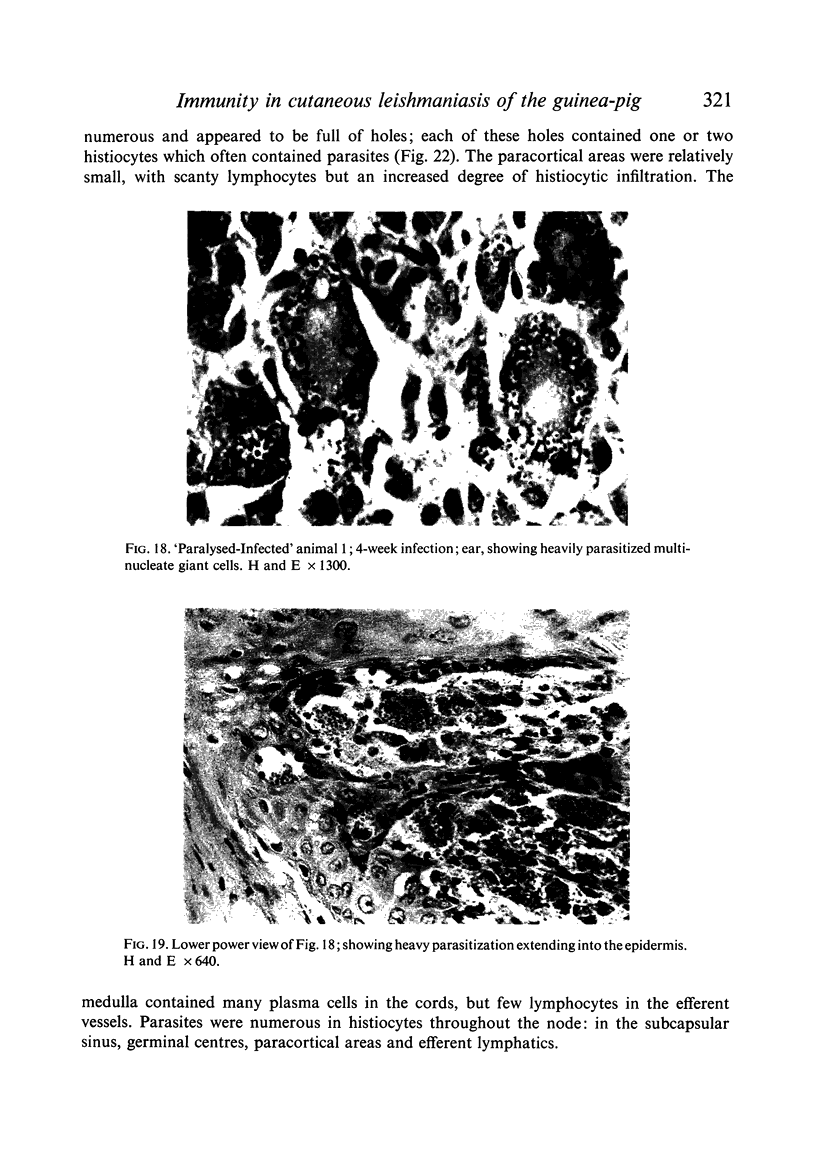
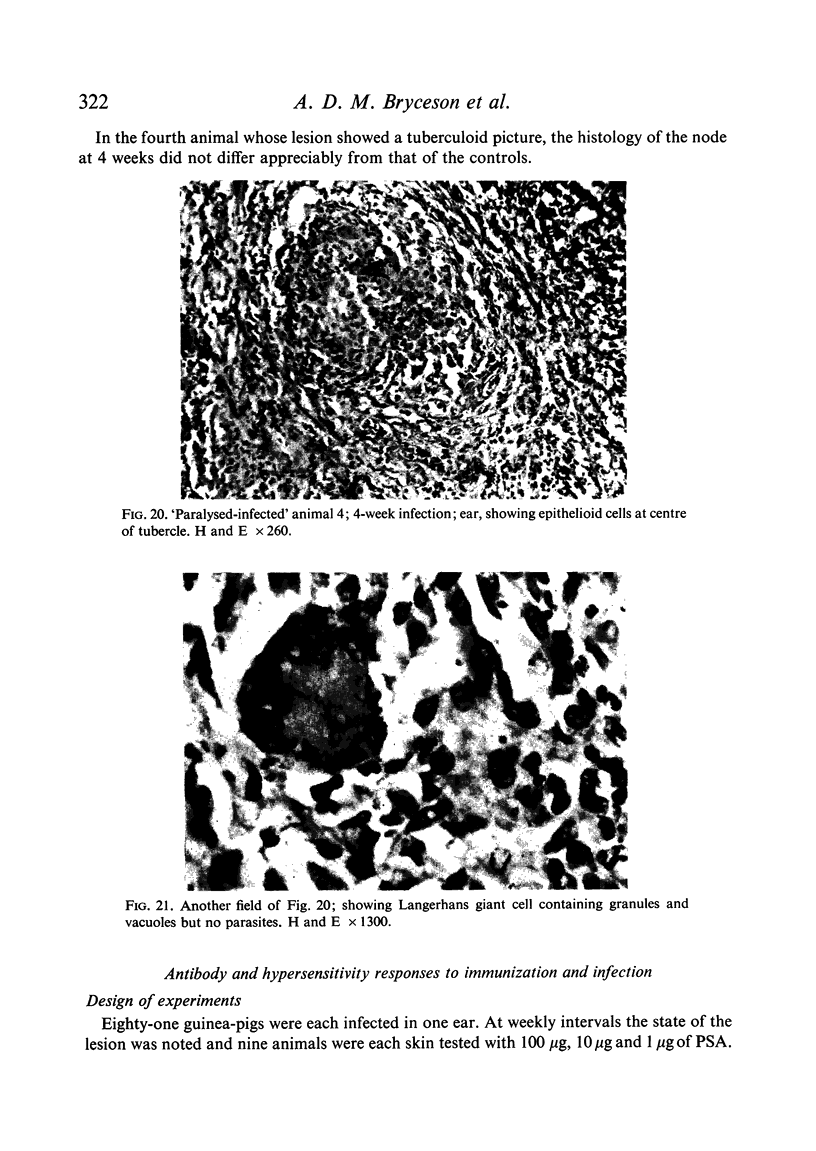
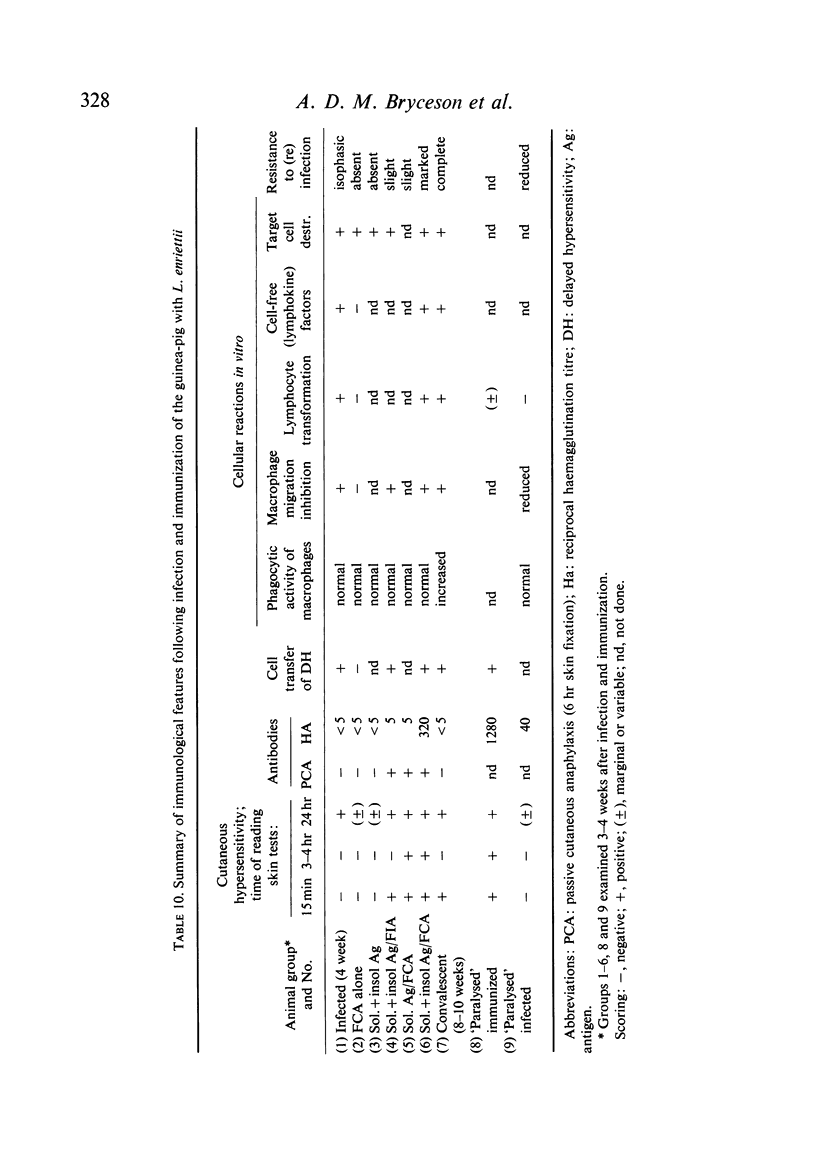
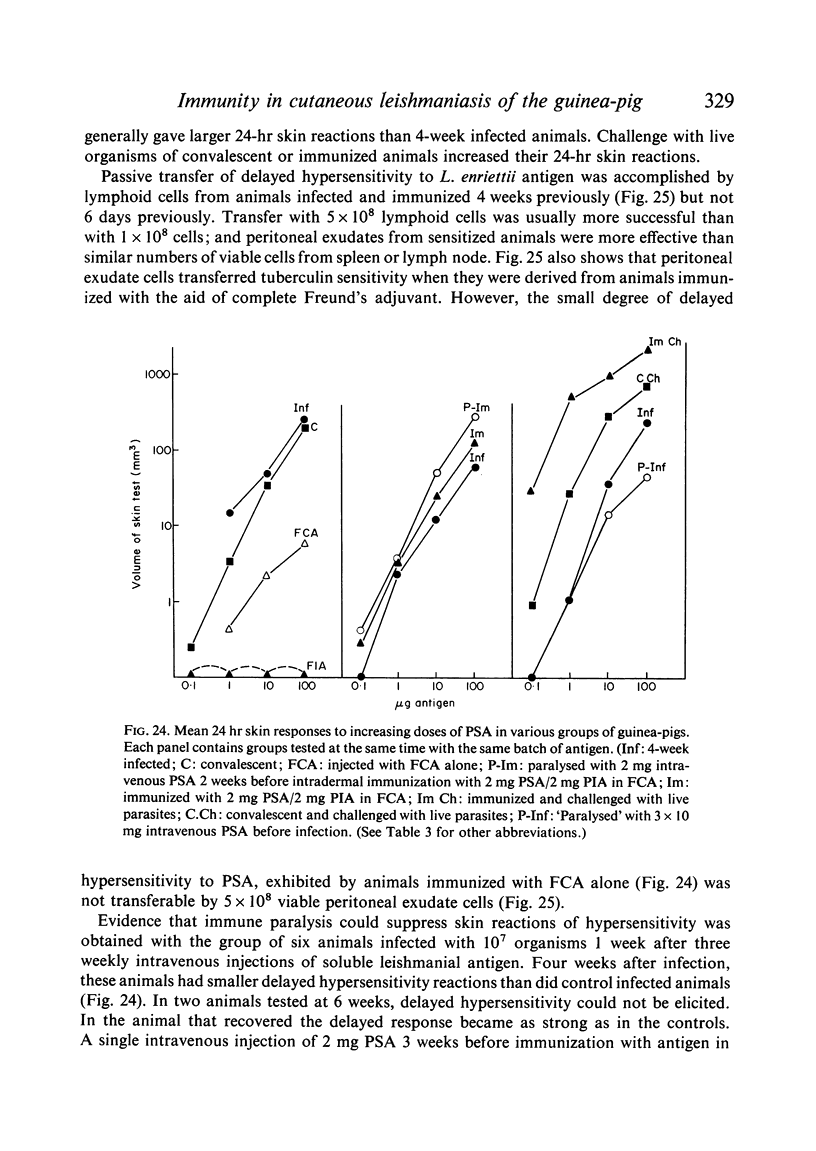
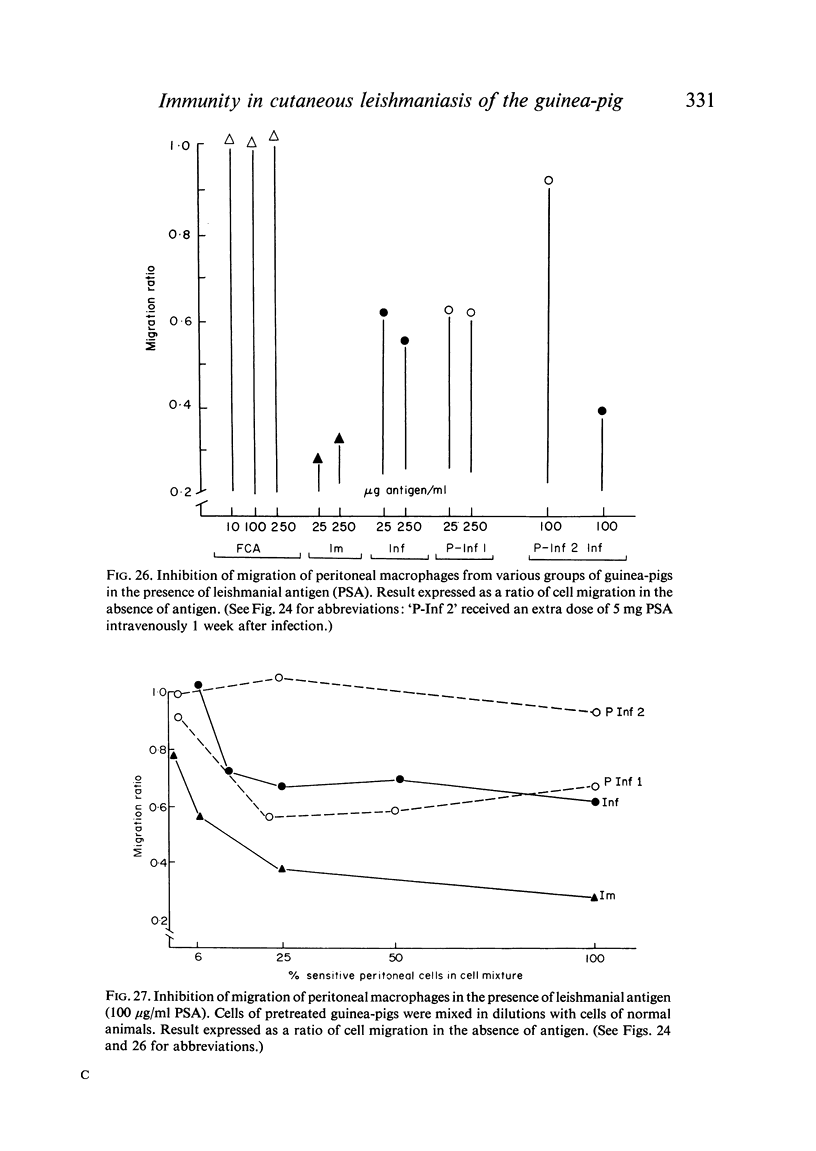
Inoculation of 106 amastigotes into the ear produced a nodule which ulcerated in 2–3 weeks and healed in 8–16 weeks. 8% of animals developed cutaneous metastases which healed with the original lesions. Histology of the primary lesions showed epidermal necrosis overlying a mass of parasitized macrophages which, after 4–6 weeks, became surrounded and infiltrated by lymphocytes. Histological changes in the draining lymph node began after 3 days and proceeded for 6 weeks; both germinal centres and paracortical areas were hyperplastic and the medulla contained many plasma cells. Superinfection produced an `isophasic' lesion, but reinfection after healing elicited only a delayed hypersensitivity response. Artificial immunization with soluble and insoluble antigenic extracts of L. enriettii in Freund's complete adjuvant partially protected against infection; extracts of other leishmanial species failed to protect. Immunological paralysis, attempted with intravenous injections of soluble antigen, increased the severity of subsequent infection.
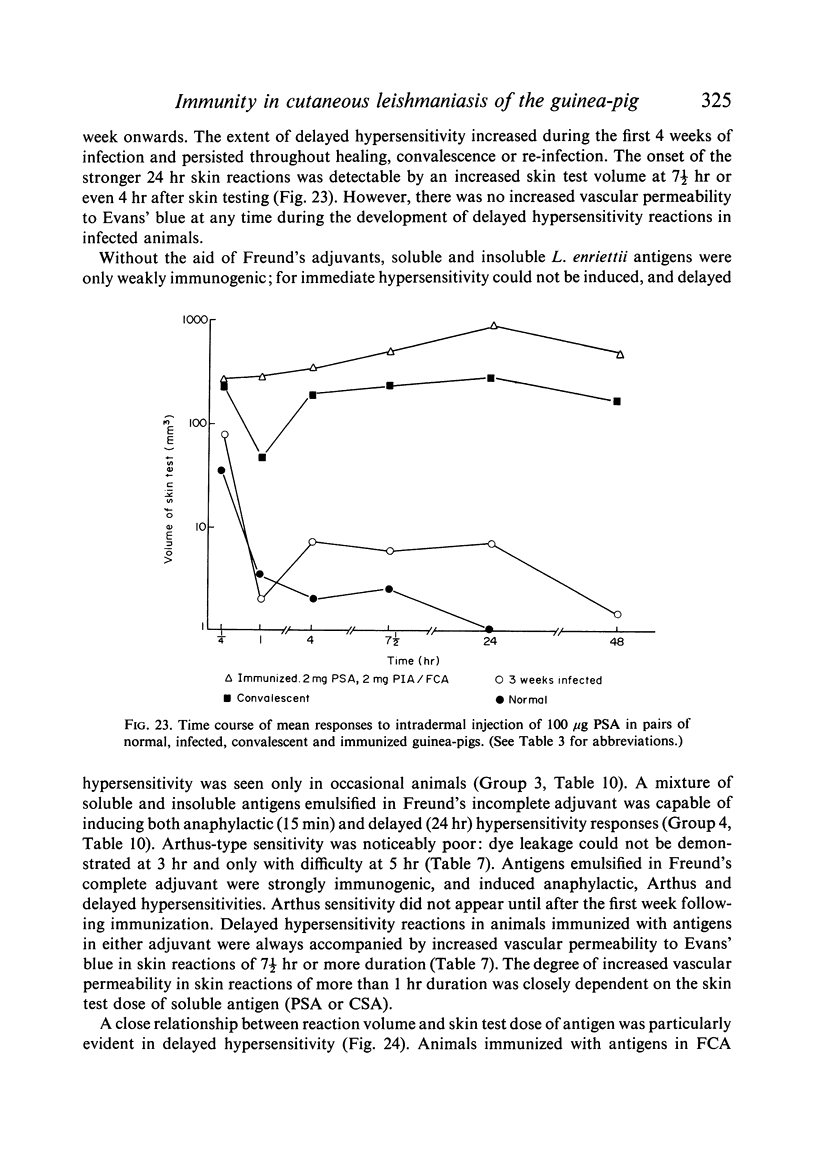
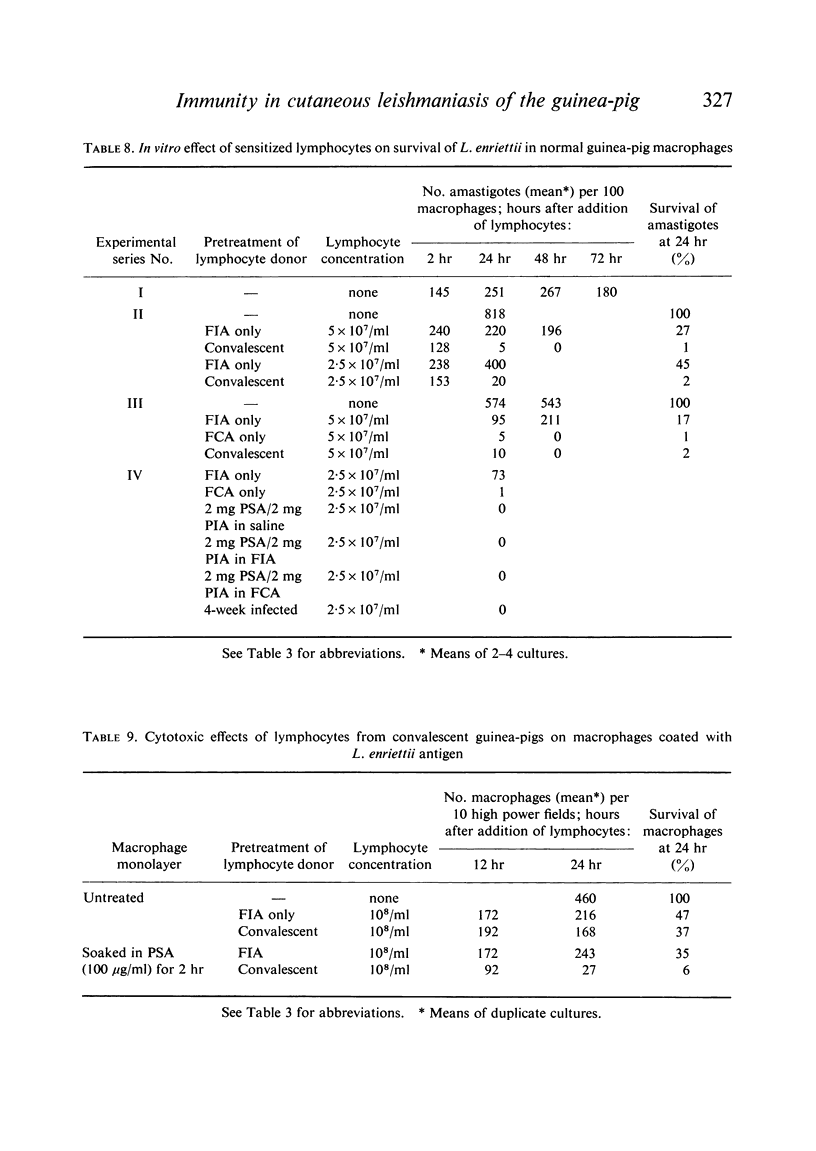
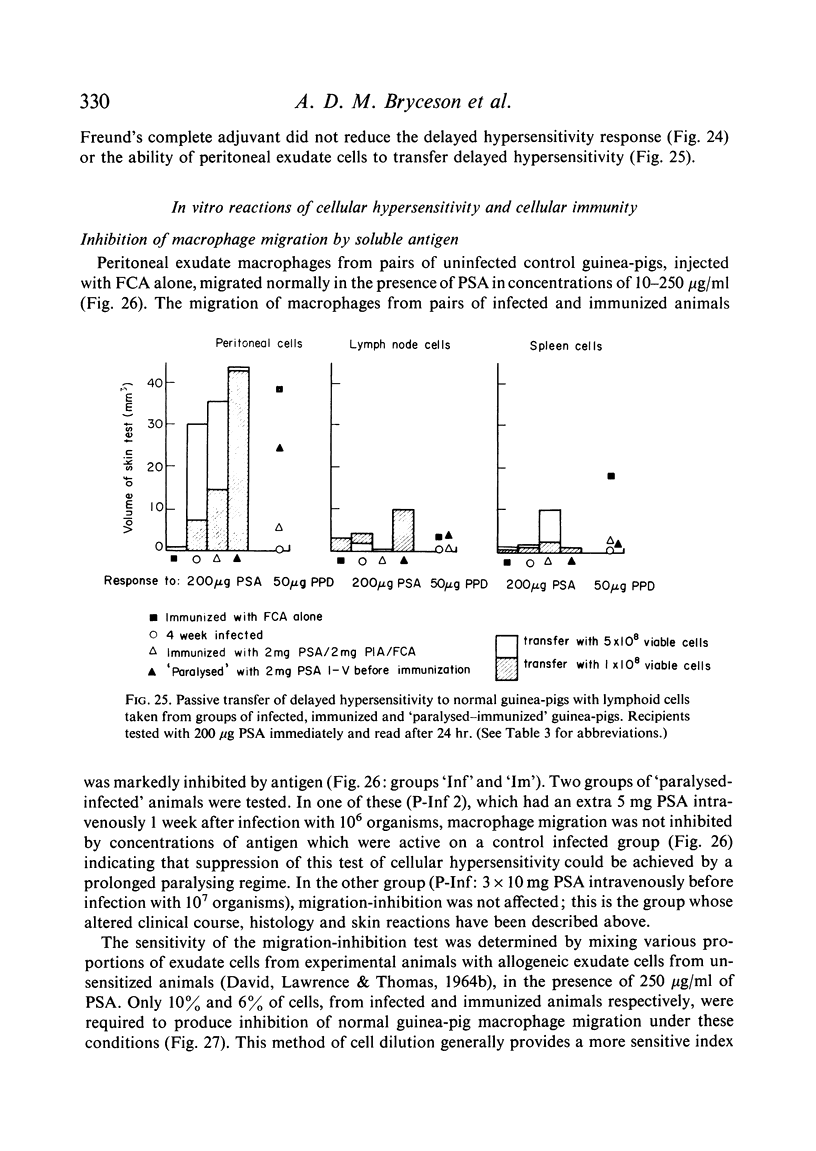
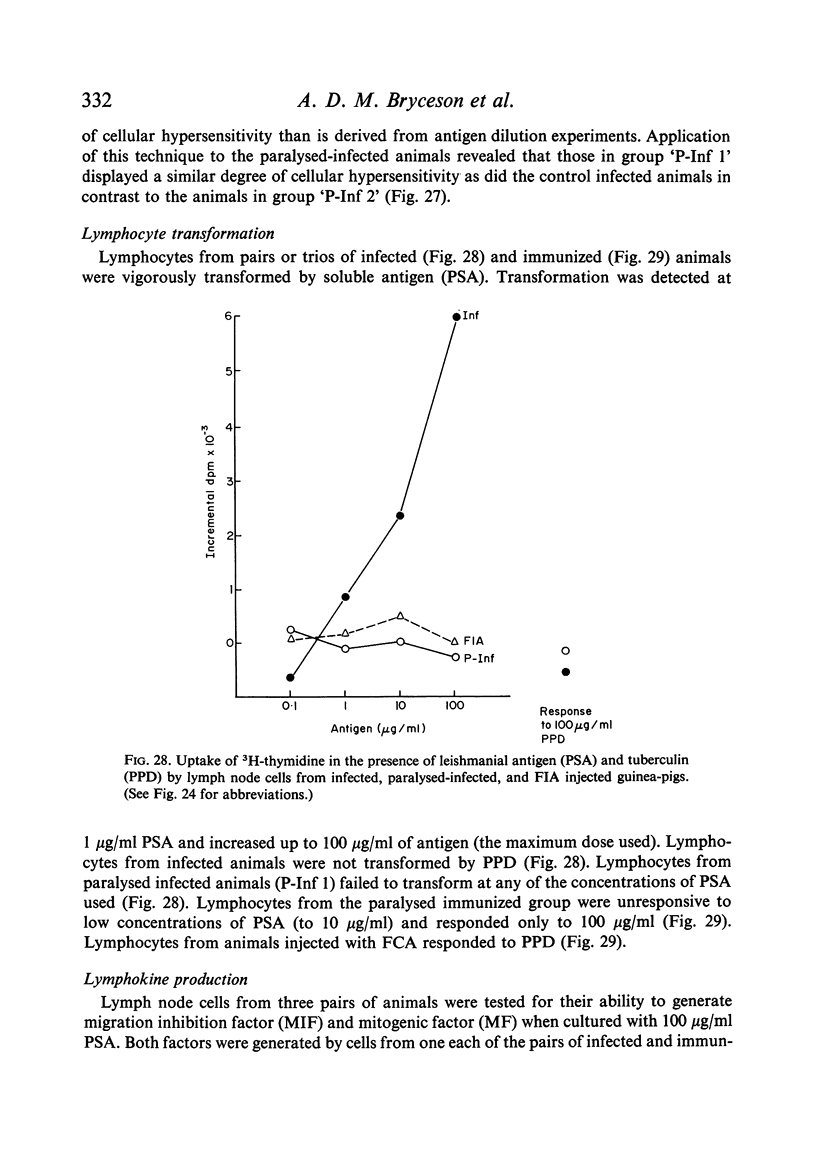
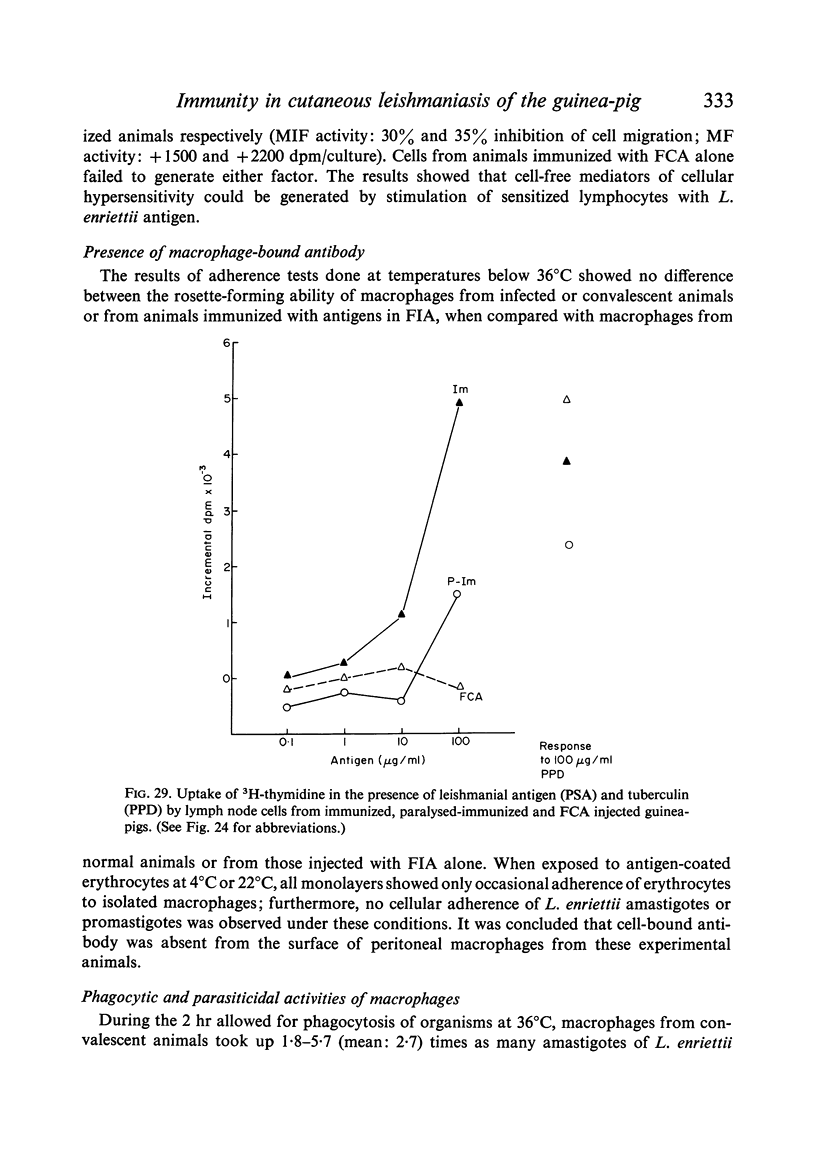
Both infection and immunization were accompanied by delayed hypersensitivity which could be transferred passively by lymphoid cells. Cell-mediated immunity was studied in vitro by the ability of soluble leishmanial antigens to transform lymphocytes, to inhibit macrophage migration, and to induce the production of lymphokine factors from lymphocytes of sensitized animals. A target cell system was devised in which sensitized lymphocytes destroyed monolayers of parasitized macrophages. Cross reactivity of leishmanial with mycobacterial antigens was shown in skin tests and in target cell destruction, but not in cell transfer or in the other cell culture systems. The phagocytic activity of peritoneal macrophages from recovered animals was increased for homologous but not for heterologous species of Leishmania; the growth of ingested organisms was not however reduced.
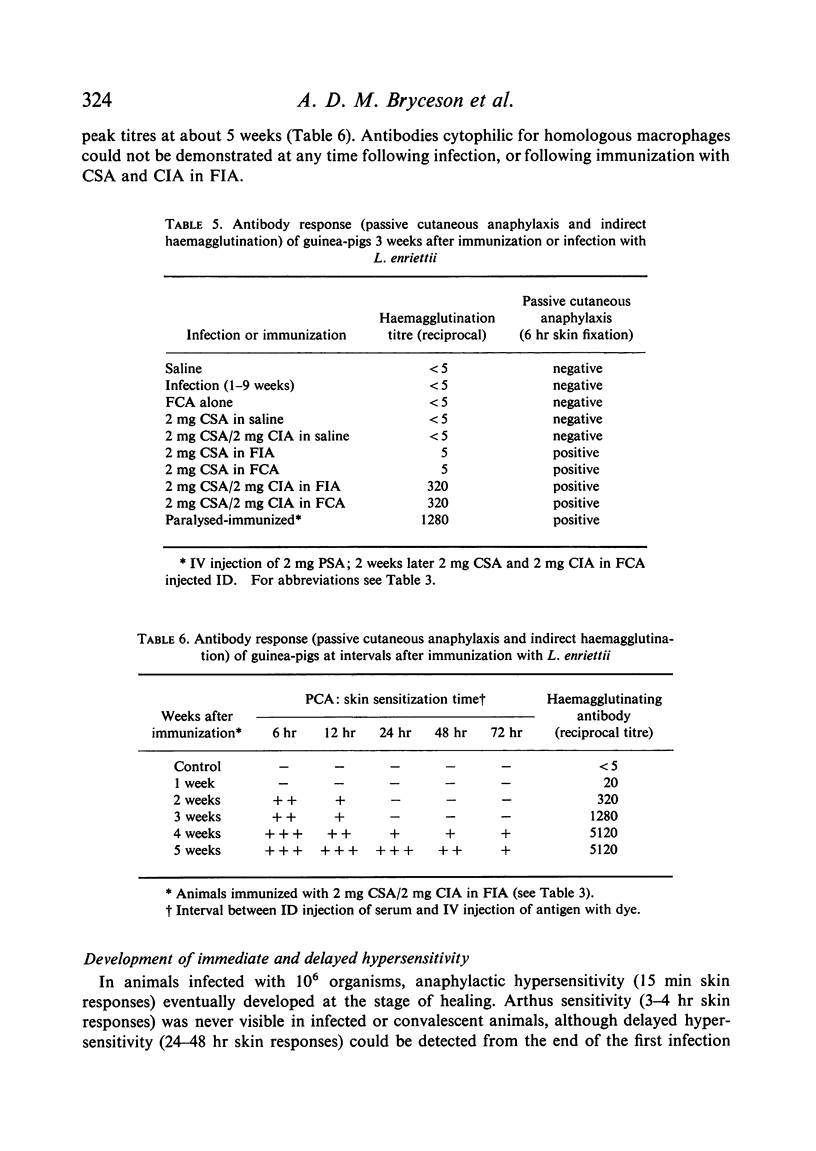
Circulating antibodies were not demonstrated by passive cutaneous anaphylaxis, or by agglutination of antigen coated sheep erythrocytes, in the sera of infected or convalescent animals, although some convalescent animals showed active cutaneous anaphylaxis. However, antibodies were demonstrated by both these techniques in immunized animals, which also showed anaphylactic and Arthus hypersensitivity when skin tested with the soluble antigens.
The results are taken to indicate that cellular mechanisms are prominent in the development of immunity of the guinea-pig against L. enriettii, and ways in which the host may eliminate the parasite are discussed. It is concluded that this model provides an experimental counterpart of human cutaneous leishmaniasis and that it is suitable for the analysis of the role of cell-mediated specific immunity in resistance to intracellular infection.
Full text
PDF




















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S., HALFF L. Observations on Leishmania enriettii Muniz and Medina, 1948. Ann Trop Med Parasitol. 1955 Mar;49(1):37–41. doi: 10.1080/00034983.1955.11685649. [DOI] [PubMed] [Google Scholar]
- BOYDEN S. V. CYTOPHILIC ANTIBODY IN GUINEA-PIGS WITH DELAYED-TYPE HYPERSENSITIVITY. Immunology. 1964 Jul;7:474–483. [PMC free article] [PubMed] [Google Scholar]
- BRAY R. S., GARNHAM P. C. The Giemsa-colophonium method for staining protozoa in tissue sections. Indian J Malariol. 1962 Jun;16:153–155. [PubMed] [Google Scholar]
- Bray R. S., Bryceson A. D. Cutaneous leishmaniasis of the guineapig. Action of sensitised lymphocytes on infected macrophages. Lancet. 1968 Oct 26;2(7574):898–899. doi: 10.1016/s0140-6736(68)91059-3. [DOI] [PubMed] [Google Scholar]
- Bray R. S., Lainson R. Studies on the immunology and serology of leishmaniasis. V. The use of particles as vehicles in passive agglutination tests. Trans R Soc Trop Med Hyg. 1967;61(4):490–505. doi: 10.1016/0035-9203(67)90099-5. [DOI] [PubMed] [Google Scholar]
- Bray R. S., Lainson R. The immunology and serology of leishmaniasis. I. The fluorescent antibody staining technique. Trans R Soc Trop Med Hyg. 1965 Sep;59(5):535–544. doi: 10.1016/0035-9203(65)90155-0. [DOI] [PubMed] [Google Scholar]
- Bray R. S., Lainson R. The immunology and serology of leishmanisis. IV. Results of Ouchterlony double diffusion tests. Trans R Soc Trop Med Hyg. 1966;60(5):605–609. doi: 10.1016/0035-9203(66)90006-x. [DOI] [PubMed] [Google Scholar]
- Bray R. S., Munford F. On the maintenance of strains of Leishmania from the Guianas. J Trop Med Hyg. 1967 Jan;70(1):23–24. [PubMed] [Google Scholar]
- Bryceson A. D. Diffuse cutaneous leishmaniasis in Ethiopia. 3. Immunological studies. IV. Pathogenesis of diffuse cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1970;64(3):380–393. doi: 10.1016/0035-9203(70)90174-4. [DOI] [PubMed] [Google Scholar]
- Bryceson A. D. Diffuse cutaneous leishmaniasis in Ethiopia. I. The clinical and histological features of the disease. Trans R Soc Trop Med Hyg. 1969;63(6):708–737. doi: 10.1016/0035-9203(69)90116-3. [DOI] [PubMed] [Google Scholar]
- CONVIT J., KERDEL-VEGAS F. DISSEMINATED CUTANEOUS LEISHMANIASIS; INNOCULATION TO LABORATORY ANIMALS, ELECTRON MICROSCOPY AND FLUORESCENT ANTIBODIES STUDIES. Arch Dermatol. 1965 May;91:439–447. doi: 10.1001/archderm.1965.01600110025007. [DOI] [PubMed] [Google Scholar]
- CONVIT J. Leishmaniasis tegumentaria difusa; nueva entidad clínico patológica y parasitaria. Rev Sanid Asist Soc. 1958 Jan-Apr;23(1-2):1–28. [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- DAVID J. R., LAWRENCE H. S., THOMAL L. DELAYED HYPERSENSITIVITY IN VITRO. II. EFFECT OF SENSITIVE CELLS ON NORMAL CELLS IN THE PRESENCE OF ANTIGEN. J Immunol. 1964 Aug;93:274–278. [PubMed] [Google Scholar]
- DOSTROVSKY A., SAGHER F., ZUCKERMAN A. [Isophasic reaction following experimental superinfection of Leishmania tropica]. AMA Arch Derm Syphilol. 1952 Dec;66(6):665–675. doi: 10.1001/archderm.1952.01530310003001. [DOI] [PubMed] [Google Scholar]
- DOSTROVSKY, ZUCKERMAN, SAGHER Successful experimental superinfection of Leishmania tropica in patients with relapsing cutaneous Leishmaniasis. Harefuah. 1952 Aug 1;43(3):29–30. [PubMed] [Google Scholar]
- Dineen J. K., Wagland B. M. The cellular transfer of immunity to Trichostrongylus colubriformis in an isogenic strain of guinea-pig. II. The relative susceptibility of the larval and adult stages of the parasite to immunological attack. Immunology. 1966 Jul;11(1):47–57. [PMC free article] [PubMed] [Google Scholar]
- Dumonde D. C. The role of the macrophage in delayed hypersensitivity. Br Med Bull. 1967 Jan;23(1):9–14. doi: 10.1093/oxfordjournals.bmb.a070524. [DOI] [PubMed] [Google Scholar]
- Dumonde D. C., Wolstencroft R. A., Panayi G. S., Matthew M., Morley J., Howson W. T. "Lymphokines": non-antibody mediators of cellular immunity generated by lymphocyte activation. Nature. 1969 Oct 4;224(5214):38–42. doi: 10.1038/224038a0. [DOI] [PubMed] [Google Scholar]
- Dávalos Mata A., Briseño Ruiz C., Flores Gasca E., Cancino Morales M. Infección experimental con cepas mexicanas del agente causal de la leishmaniasis cutánea. Salud Publica Mex. 1968 Mar-Apr;10(2):159–171. [PubMed] [Google Scholar]
- Garnham P. C., Humphrey J. H. Problems in leishmaniasis related to immunology. Curr Top Microbiol Immunol. 1969;48:29–42. doi: 10.1007/978-3-642-46163-7_2. [DOI] [PubMed] [Google Scholar]
- Glazunova Z. I. Allergicheskie reaktsii u morskikh svinok pri povtornom zarazhenii Leishmania enrietti. Med Parazitol (Mosk) 1965 Sep-Oct;34(5):582–585. [PubMed] [Google Scholar]
- Kolb W. P., Granger G. A. Lymphocyte in vitro cytotoxicity: characterization of human lymphotoxin. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1250–1255. doi: 10.1073/pnas.61.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmar W. Immunität bei der Leishmania enriettii-Infektion des Meerschweinchens. Z Tropenmed Parasitol. 1965 Oct;16(3):277–283. [PubMed] [Google Scholar]
- LAINSON R., STRANGWAYS-DIXON J. LEISHMANI MEXICANA: THE EPIDEMIOLOGY OF DERMAL LEISHMANIASIS IN BRITISH HONDURAS. Trans R Soc Trop Med Hyg. 1963 Jul;57:242–265. doi: 10.1016/0035-9203(63)90182-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lainson R., Bray R. S. Studies on the immunology and serology of leishmaniasis. II. Cross-immunity experiments among different forms of American cutaneous leishmaniasis in monkeys. Trans R Soc Trop Med Hyg. 1966;60(4):526–532. doi: 10.1016/0035-9203(66)90278-1. [DOI] [PubMed] [Google Scholar]
- MANSON-BAHR P. E., HEISCH R. B., GARNHAM P. C. Studies in leishmanifasis in East Africa. IV. The Montenegro test in kala-azar in Kenya. Trans R Soc Trop Med Hyg. 1959 Sep;53:380–383. doi: 10.1016/0035-9203(59)90038-0. [DOI] [PubMed] [Google Scholar]
- MANSON-BAHR P. E., SOUTHGATE B. A. RECENT RESEARCH ON KALA AZAR IN EAST AFRICA. J Trop Med Hyg. 1964 Apr;67:79–84. [PubMed] [Google Scholar]
- MEDINA R., ROMERO J. [Clinical and parasitological study of a new strain of Leishmania]. Arch Venez Med Trop Parasitol Med. 1959 Jul;3:298–326. [PubMed] [Google Scholar]
- Medawar P. B. The behaviour and fate of skin autografts and skin homografts in rabbits: A report to the War Wounds Committee of the Medical Research Council. J Anat. 1944 Oct;78(Pt 5):176–199. [PMC free article] [PubMed] [Google Scholar]
- Miller H. C., Twohy D. W. Cellular immunity to Leishmania donovani in macrophages in cultures. J Parasitol. 1969 Feb;55(1):200–207. [PubMed] [Google Scholar]
- NEAL R. A. CHEMOTHERAPY OF CUTANEOUS LEISHMANIASIS: LEISHMANIA TROPICA INFECTIONS IN MICE. Ann Trop Med Parasitol. 1964 Dec;58:420–430. doi: 10.1080/00034983.1964.11686264. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Wolstencroft R. A., Gell P. G. Delayed hypersensitivity in the guinea-pig to a protein-hapten conjugate and its relationship to in vitro transformation of lymph node, spleen, thymus and peripheral blood lymphocytes. Immunology. 1967 Jan;12(1):89–102. [PMC free article] [PubMed] [Google Scholar]
- PARAENSE W. L. The spread of Leishmania enriettii through the body of the guineapig. Trans R Soc Trop Med Hyg. 1953 Nov;47(6):556–560. doi: 10.1016/s0035-9203(53)80008-8. [DOI] [PubMed] [Google Scholar]
- Stauber L. A. Characterization of strains of Leishmania donovani. Exp Parasitol. 1966 Feb;18(1):1–11. doi: 10.1016/0014-4894(66)90002-6. [DOI] [PubMed] [Google Scholar]
- Vasina S. G., Demina N. A., Glazunova Z. I. Morfologicheskoe i tsitokhimicheskoe izuchenie limfaticheskikh uzlov i selezenki pri leishmanioze morskikh svinok (Leishmania enrietti) Med Parazitol (Mosk) 1965 Nov-Dec;34(6):708–713. [PubMed] [Google Scholar]
- Willms-Kretschmer K., Flax M. H., Cotran R. S. The fine structure of the vascular response in hapten-specific delayed hypersensitivity and contact dermatitis. Lab Invest. 1967 Sep;17(3):334–349. [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Recent studies on acquired immunity in tuberculosis. Curr Top Microbiol Immunol. 1969;48:129–178. doi: 10.1007/978-3-642-46163-7_6. [DOI] [PubMed] [Google Scholar]