Abstract
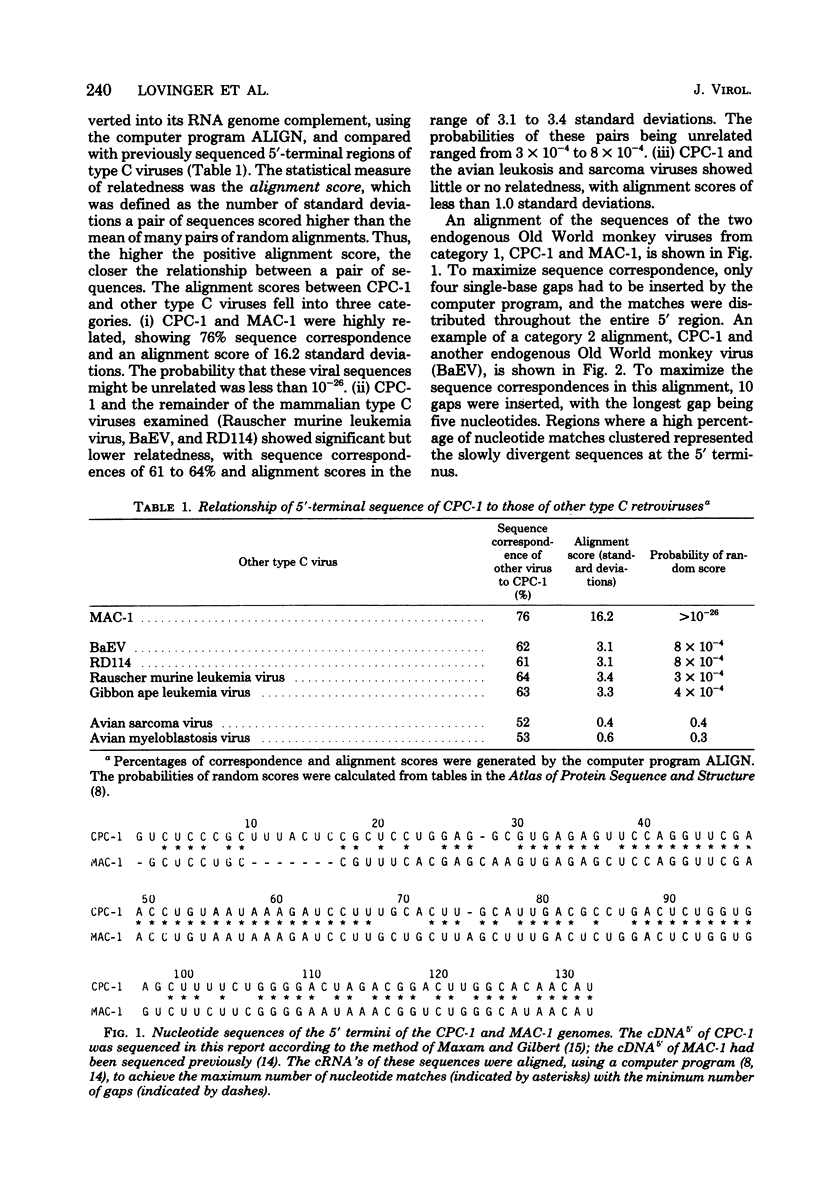
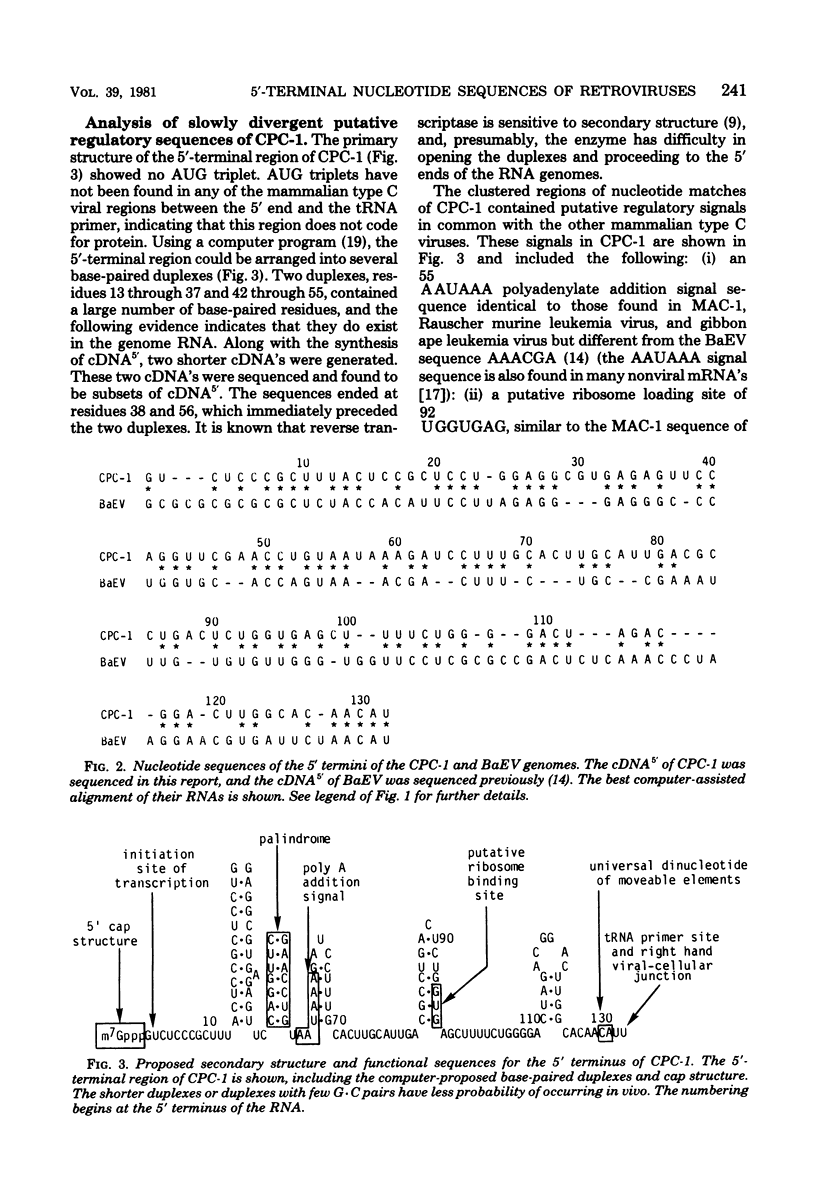
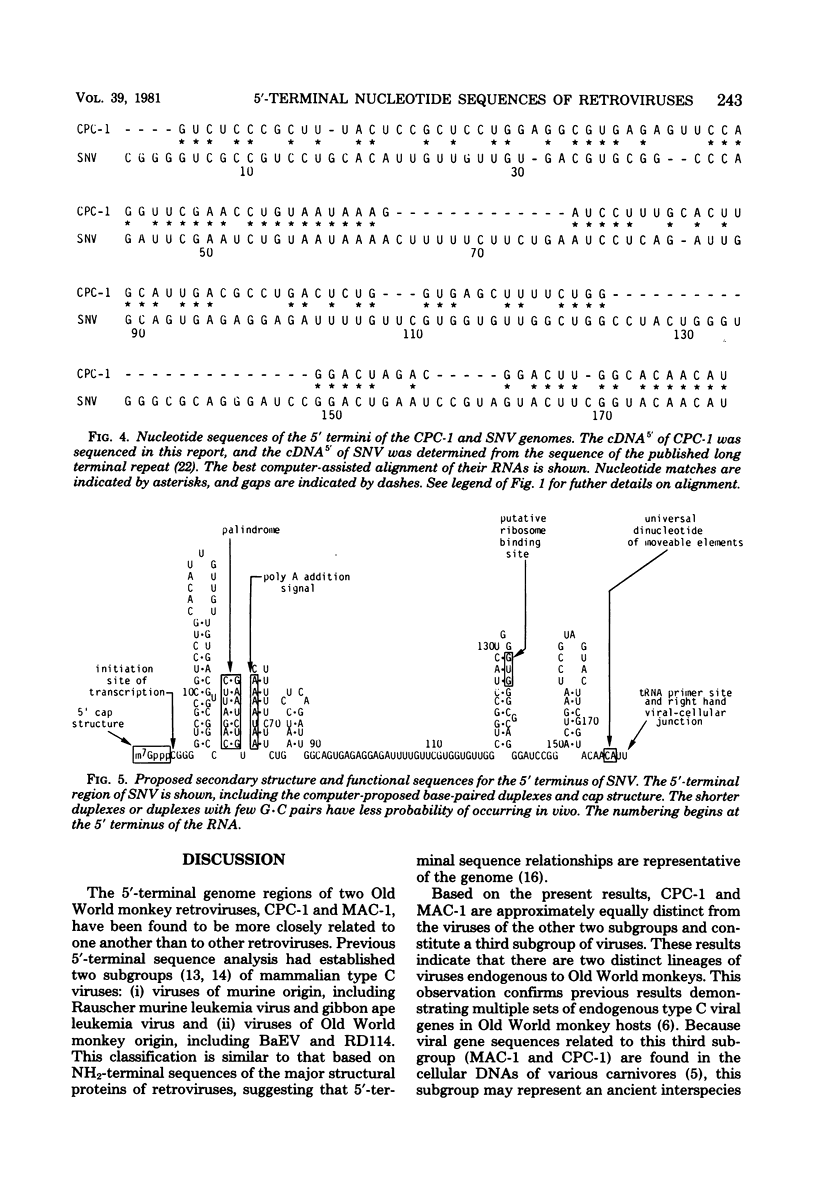
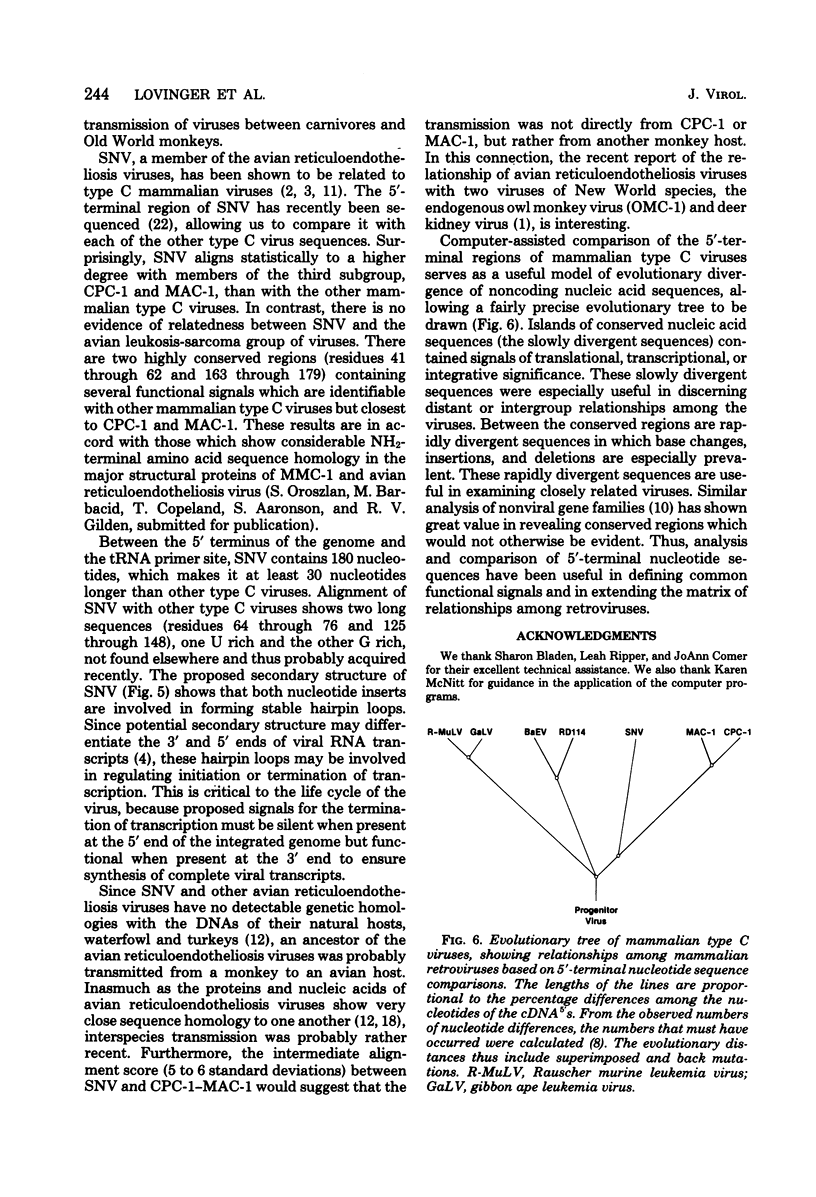
Computer-assisted comparison of the 5′-terminal regions of mammalian type C viruses serves as a useful model of evolutionary divergence of noncoding nucleic acid sequences. It has led to the concept that regions of conserved nucleic acid sequences, the slowly divergent sequences, contain signals of translational, transcriptional, or integrative significance. Interspersed among the conserved regions are rapidly divergent sequences in which base changes, insertions, and deletions are especially prevalent. In the present study, CPC-1, a type C virus isolated from Colobus polykomos, was shown to be related to another Old World type C monkey virus, endogenous stump-tailed monkey virus, MAC-1, by analysis of their 5′-terminal nucleotide sequences. The 5′-terminal regions of CPC-1 and MAC-1 showed a 76% nucleotide correspondence and were of similar lengths, 132 and 127 nucleotides, respectively. Previous strong-stop analyses of other type C viruses have defined two subgroups: (i) Rauscher murine leukemia virus and gibbon ape leukemia virus and (ii) baboon endogenous virus and endogenous cat virus RD114. Based on the present sequence analysis of their 5′-terminal sequences, CPC-1 and MAC-1 formed a third subgroup. Computer-assisted comparison of the 5′-terminal sequences of CPC-1 and MAC-1 to the previously reported sequence of avian spleen necrosis virus (SNV) (Shimotohno et al., Nature [London] 285:550-554, 1980) showed SNV to be a member of that subgroup of mammalian type C viruses. Consistent with the inclusion of SNV in this subgroup of mammalian type C viruses, SNV was distantly related to other mammalian type C viruses. Interestingly, the SNV 5′-terminal sequences showed no significant evolutionary relationship by these criteria to the avian leukemia and sarcoma viruses. CPC-1, MAC-1, and SNV contained conserved regulatory signals in similar positions in their 5′-terminal RNA sequences analogous to those observed in other mammalian type C retroviruses. These sequences included the canonical AAUAAA sequence, a palindrome, a putative ribosome binding site, and an integration site. Some of these highly conserved subsequences were common to 3′- and 5′-terminal noncoding sequences of nonviral eucaryotic mRNA's (Efstratiadis et al., Cell 21:653-668, 1980). Thus, analysis and comparison of 5′-terminal nucleotide sequences have been useful in defining common functional signals and in extending the matrix of relationships among retroviruses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Daniel M. D., Aaronson S. A. Immunological relationships of OMC-1, an endogenous virus of owl monkeys, with mammalian and avian type C viruses. J Virol. 1980 Jan;33(1):561–566. doi: 10.1128/jvi.33.1.561-566.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Hunter E., Aaronson S. A. Avian reticuloendotheliosis viruses: evolutionary linkage with mammalian type C retroviruses. J Virol. 1979 May;30(2):508–514. doi: 10.1128/jvi.30.2.508-514.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G., Temin H. M. Specific antigenic relationships between the RNA-dependent DNA polymerases of avian reticuloendotheliosis viruses and mammalian type C retroviruses. J Virol. 1980 Apr;34(1):168–177. doi: 10.1128/jvi.34.1.168-177.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz E. W., Jr, Wydro R. M., Nadal-Ginard B., Dina D. Moloney murine sarcoma proviral DNA is a transcriptional unit. Nature. 1980 Dec 25;288(5792):665–669. doi: 10.1038/288665a0. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Todaro G. J. Carnivores have sequences in their cellular DNA distantly related to the primate endogenous virus, MAC-1. Virology. 1979 Apr 15;94(1):224–227. doi: 10.1016/0042-6822(79)90454-9. [DOI] [PubMed] [Google Scholar]
- Bryant M. L., Sherr C. J., Sen A., Todaro G. J. Molecular diversity among five different endogenous primate retroviruses. J Virol. 1978 Oct;28(1):300–313. doi: 10.1128/jvi.28.1.300-313.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman H. P., Gilden R. V., Oroszlan S. Reticuloendotheliosis virus: detection of immunological relationship to mammalian type C retroviruses. J Virol. 1979 Mar;29(3):1221–1225. doi: 10.1128/jvi.29.3.1221-1225.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Hunter E., Bhown A. S., Bennett J. C. Amino-terminal amino acid sequence of the major structural polypeptides of avian retroviruses: sequence homology between reticuloendotheliosis virus p30 and p30s of mammalian retroviruses. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2708–2712. doi: 10.1073/pnas.75.6.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Reticuloendotheliosis virus nucleic acid sequences in cellular DNA. J Virol. 1974 Nov;14(5):1179–1188. doi: 10.1128/jvi.14.5.1179-1188.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger G. G., Schochetman G. 5' terminal nucleotide sequences of type C retroviruses: features common to noncoding sequences of eucaryotic messenger RNAs. Cell. 1980 Jun;20(2):441–449. doi: 10.1016/0092-8674(80)90630-3. [DOI] [PubMed] [Google Scholar]
- Lovinger G. G., Schochetman G. 5'-terminal nucleotide sequences of the Rauscher leukemia virus and gibbon ape leukemia virus genomes exhibit a high degree of correspondence. J Virol. 1979 Dec;32(3):803–811. doi: 10.1128/jvi.32.3.803-811.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Witter R. L. The reticuloendotheliosis viruses. Curr Top Microbiol Immunol. 1975;71:103–124. doi: 10.1007/978-3-642-66193-8_3. [DOI] [PubMed] [Google Scholar]
- Queen C. L., Korn L. J. Computer analysis of nucleic acids and proteins. Methods Enzymol. 1980;65(1):595–609. doi: 10.1016/s0076-6879(80)65062-9. [DOI] [PubMed] [Google Scholar]
- Rabin H., Benton C. V., Tainsky M. A., Rice N. R., Gilden R. V. Isolation and characterization of an endogenous type C virus of rhesus monkeys. Science. 1979 May 25;204(4395):841–842. doi: 10.1126/science.87013. [DOI] [PubMed] [Google Scholar]
- Sherwin S. A., Todaro G. J. A new endogenous primate type C virus isolated from the Old World monkey Colobus polykomos. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5041–5045. doi: 10.1073/pnas.76.10.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Origin of retroviruses from cellular moveable genetic elements. Cell. 1980 Oct;21(3):599–600. doi: 10.1016/0092-8674(80)90420-1. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Sherwin S. A., Sherr C. J. MAC-1, a new genetically transmitted type C virus of primates: "low frequency" activation from stumptail monkey cell cultures. Cell. 1978 Apr;13(4):775–782. doi: 10.1016/0092-8674(78)90227-1. [DOI] [PubMed] [Google Scholar]


