Abstract
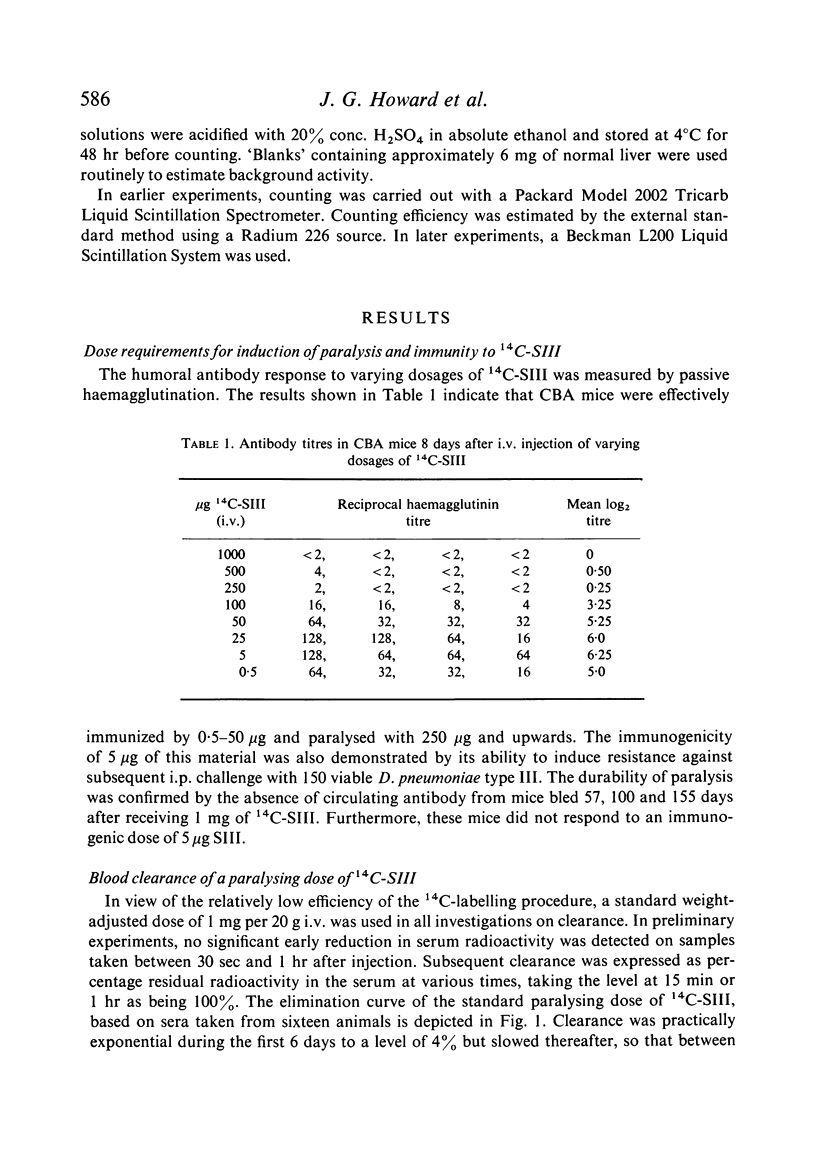
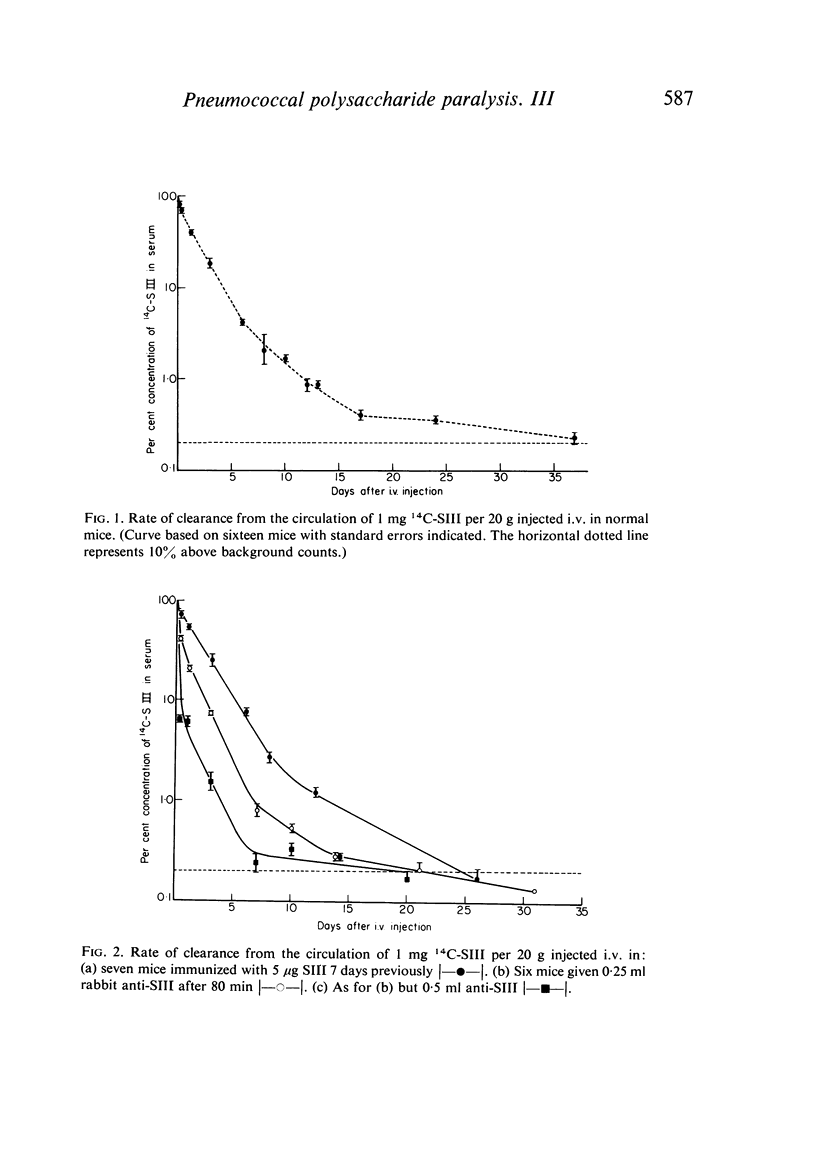
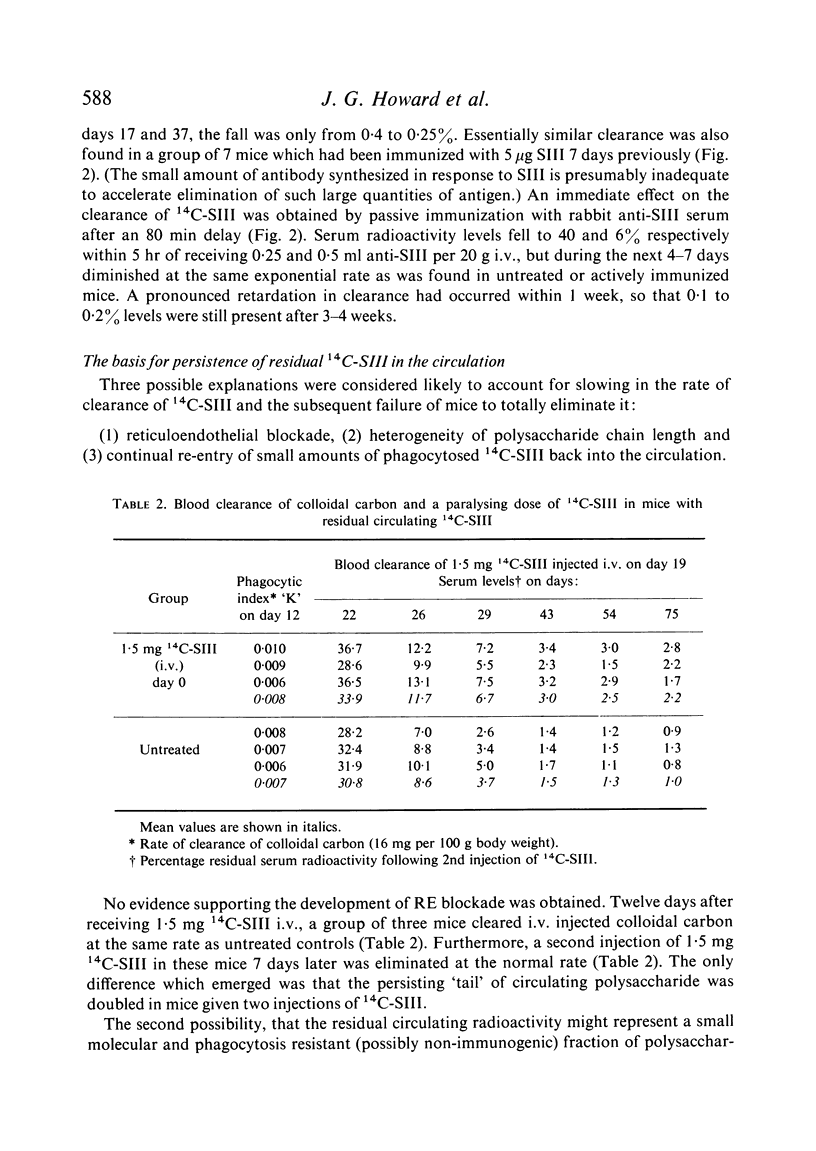
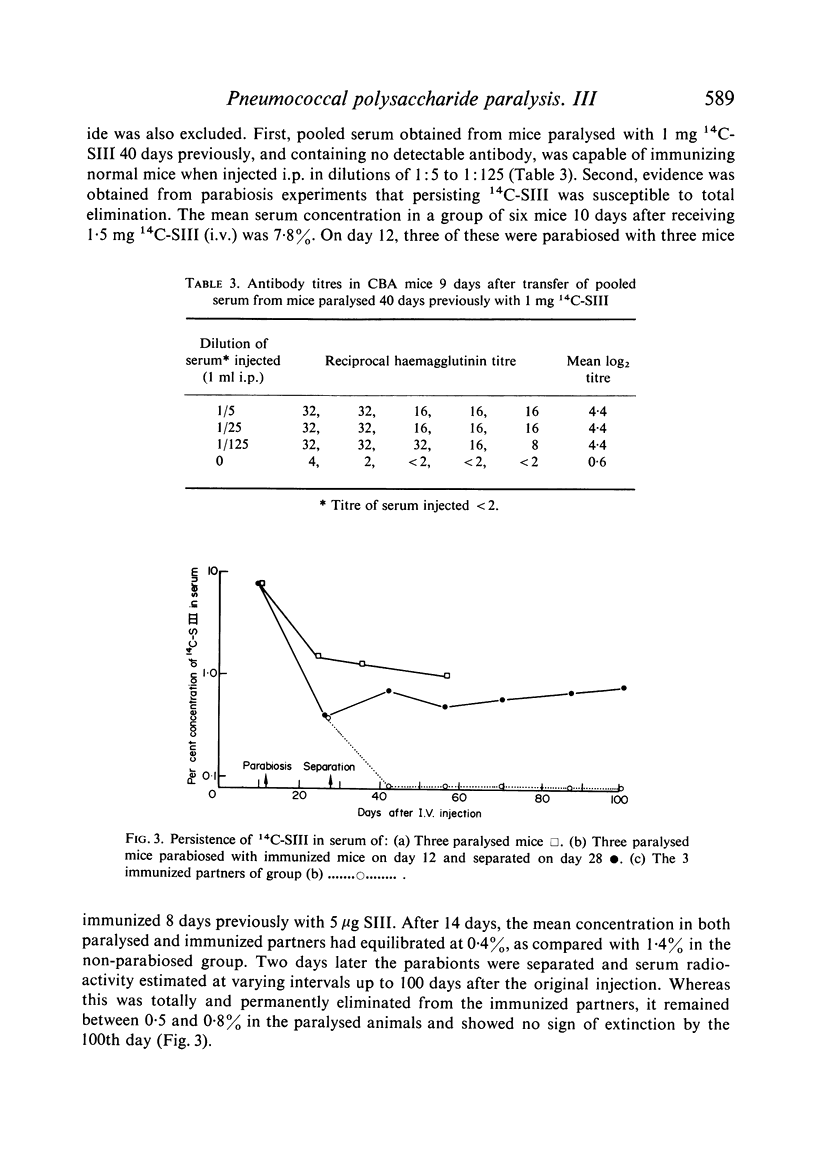
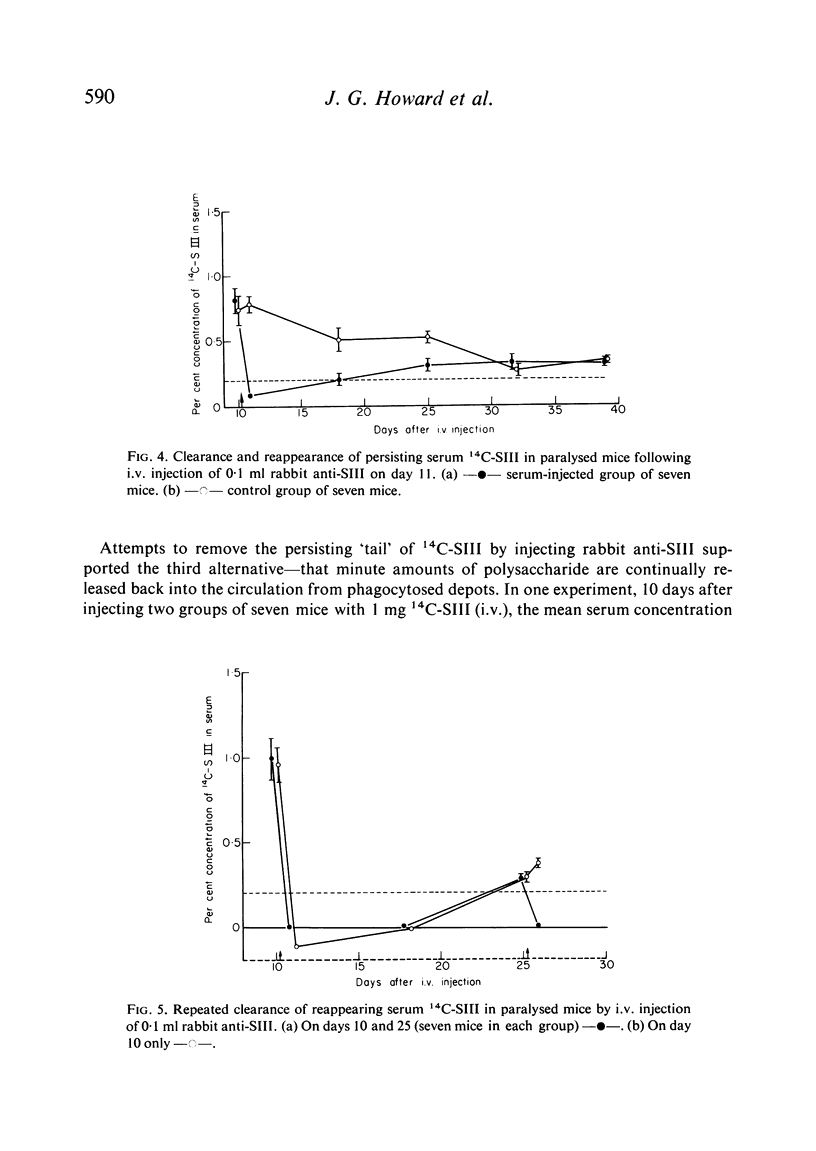
Clearance of a paralysing dose of 14C-labelled type III pneumococcal polysaccharide from the circulation in CBA mice was found to be exponential for about 6 days, but slowed thereafter. A persisting `tail' of free immunogenic 14C-SIII remained at levels between 0·1–1%, even after 100 days. This was not prevented by active or passive immunization, although the latter promoted early clearance. The mechanism responsible for this heterogeneity of elimination was investigated. Normal clearance of colloidal carbon and a second dose of 14C-SIII excluded reticuloendothelial blockade. The application of parabiosis followed by separation excluded the presence of a phagocytosis-resistant fraction of 14C-SIII. Continual leakage of phagocytosed 14C-SIII into the circulation was implicated by its gradual reappearance after total elimination by anti-SIII, a procedure which could be repeated.
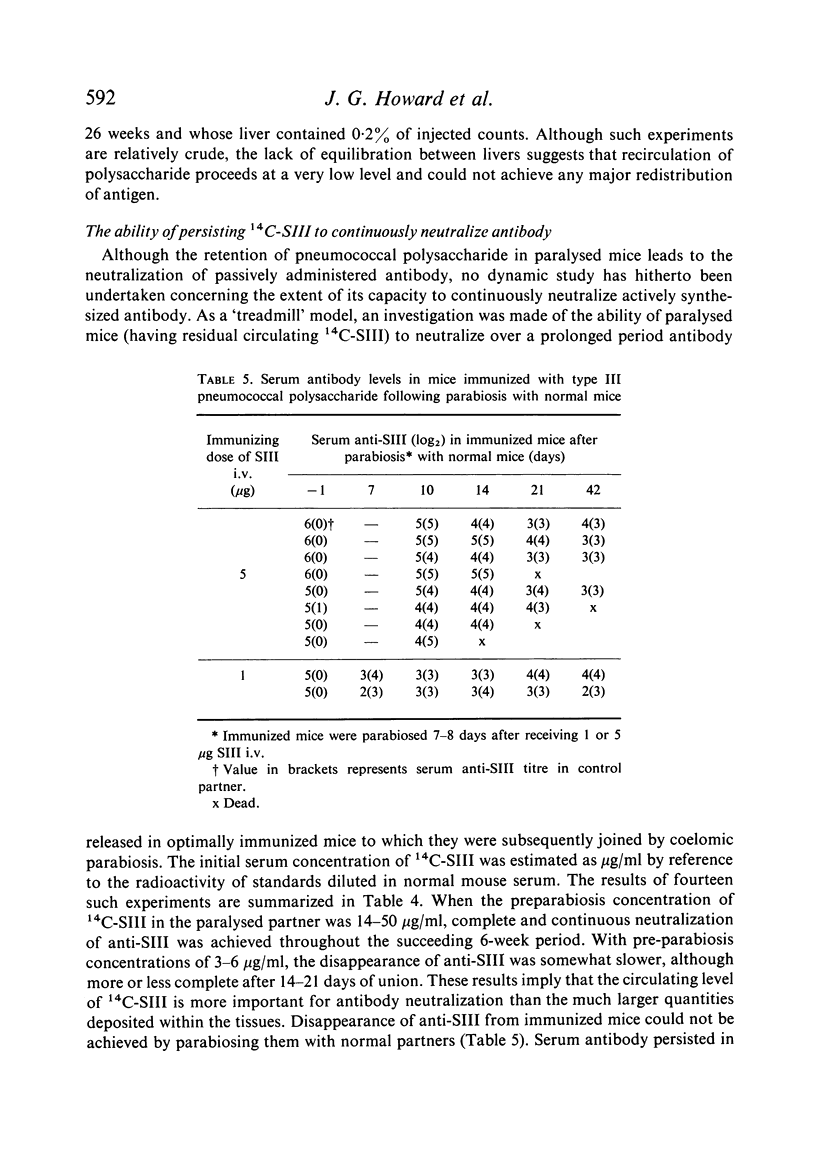
The ability of recirculating 14C-SIII to continuously neutralize antibody synthesis on a `treadmill' basis was investigated by parabiosis of paralysed to optimally-immunized mice. Serum antibody was eliminated throughout 6 weeks of union. The data implied that serum 14C-SIII levels rather than tissue depots largely determined antibody neutralization. The contribution of this mechanism to polysaccharide paralysis is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., FARTHING C. P., HUMPHREY J. H. The significance of multiple antibody components in serum of immunized rabbits. Immunology. 1960 Oct;3:336–351. [PMC free article] [PubMed] [Google Scholar]
- Adlersberg L., Singer J. M., Ende E. Redistribution and elimination of intravenously injected latex particles in mice. J Reticuloendothel Soc. 1969 Oct-Dec;6(5):536–560. [PubMed] [Google Scholar]
- BIOZZI G., BENACERRAF B., STIFFEL C., HALPERN B. N. Etude quantitative de l'activité granulopexique du système réticuloendothéliai chez la souris. C R Seances Soc Biol Fil. 1954 Mar;148(5-6):431–435. [PubMed] [Google Scholar]
- DIXON F. J., MAURER P. H., WEIGLE W. O. Immunologic activity of pneumococcal polysaccharide fixed in the tissues of the mouse. J Immunol. 1955 Mar;74(3):188–191. [PubMed] [Google Scholar]
- FELTON L. D., PRESCOTT B., KAUFFMANN G., OTTINGER B. Pneumococcal antigenic polysaccharide substances from animal tissues. J Immunol. 1955 Mar;74(3):205–213. [PubMed] [Google Scholar]
- Howard J. G., Elson J., Christie G. H., Kinsky R. G. Studies on immunological paralysis. II. The detection and significance of antibod-forming cells in the spleen during immunological paralysis with type 3 pneumococcal polysaccharide. Clin Exp Immunol. 1969 Jan;4(1):41–53. [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Siskind G. W. Studies on immunological paralysis. I. A consideration of macrophage involvement in the induction of paralysis and immunity by type II pneumococcal polysaccharide. Clin Exp Immunol. 1969 Jan;4(1):29–39. [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Humphrey J. H. Synthetic antigens composed exclusively of L- or D- amino acids. II. Effect of optical configuration on the metabolism and fate of synthetic polypeptide antigens in mice. Immunology. 1968 Feb;14(2):225–234. [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Humphrey J. H. The fate of a D-amino acid polypeptide [p(D-Tyr, D-Glu, D-Ala), 247] in newborn and adult mice: relationship to the induction of tolerance. Isr J Med Sci. 1969 Mar-Apr;5(2):185–195. [PubMed] [Google Scholar]
- KAPLAN M. E., COONS A. H., DEANE H. W. Localization of antigen in tissue cells; cellular distribution of pneumococcal polysaccharides types II and III in the mouse. J Exp Med. 1950 Jan 1;91(1):15-30, 4 pl. doi: 10.1084/jem.91.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsky R. G., Christie G. H., Elson J., Howard J. G. Extra-hepatic derivation of Kupffer cells during oestrogenic stimulation of parabiosed mice. Br J Exp Pathol. 1969 Oct;50(5):438–447. [PMC free article] [PubMed] [Google Scholar]
- SISKIND G. W., PATERSON P. Y. A BIOASSAY FOR ESTIMATION OF PNEUMOCOCCAL POLYSACCHARIDE IN UNRESPONSIVE (PARALYZED) MICE. J Immunol. 1964 Sep;93:489–494. [PubMed] [Google Scholar]
- SISKIND G. W., PATERSON P. Y., THOMAS L. INDUCTION OF UNRESPONSIVENESS AND IMMUNITY IN NEWBORN AND ADULT MICE WITH PNEUMOCOCCAL POLYSACCHARIDE. J Immunol. 1963 Jun;90:929–934. [PubMed] [Google Scholar]
- STARK O. K. Studies on pneumococcal polysaccharide. II. Mechanism involved in production of immunological paralysis by type I pneumococcal polysaccharide. J Immunol. 1955 Feb;74(2):130–133. [PubMed] [Google Scholar]


