Abstract
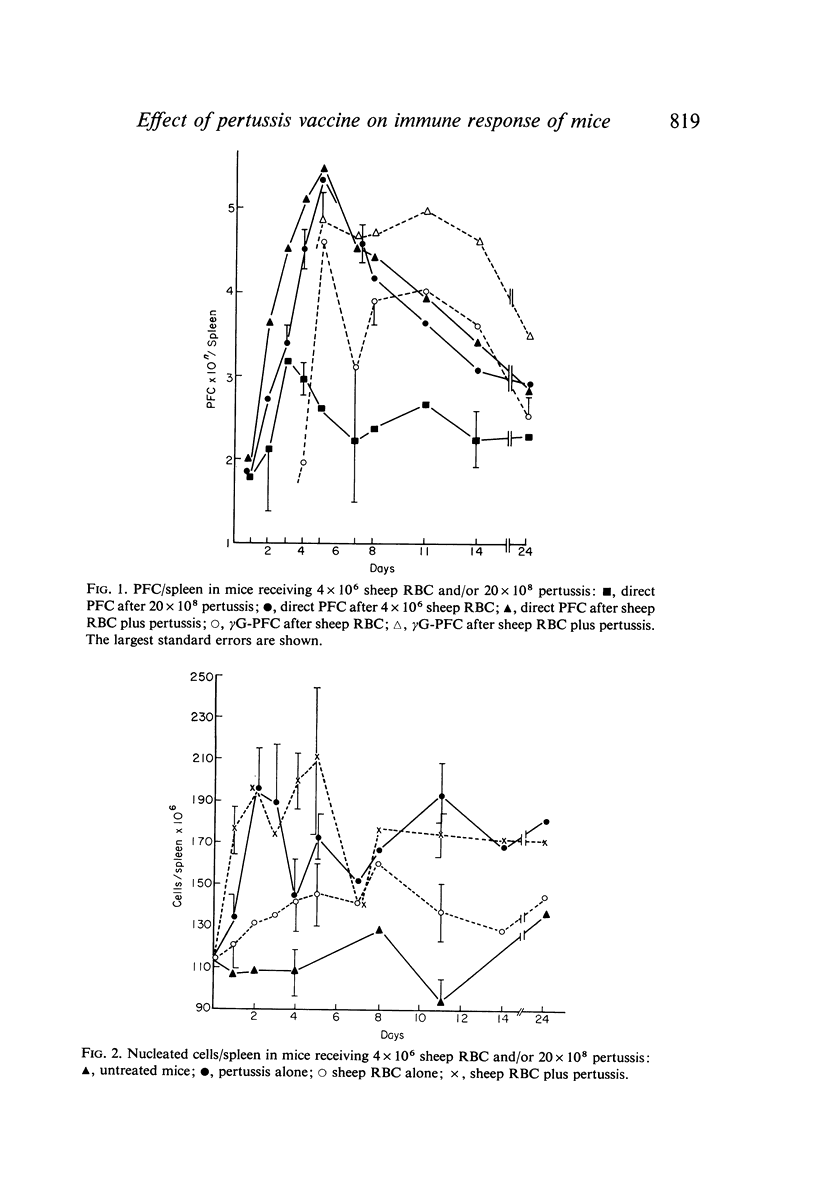
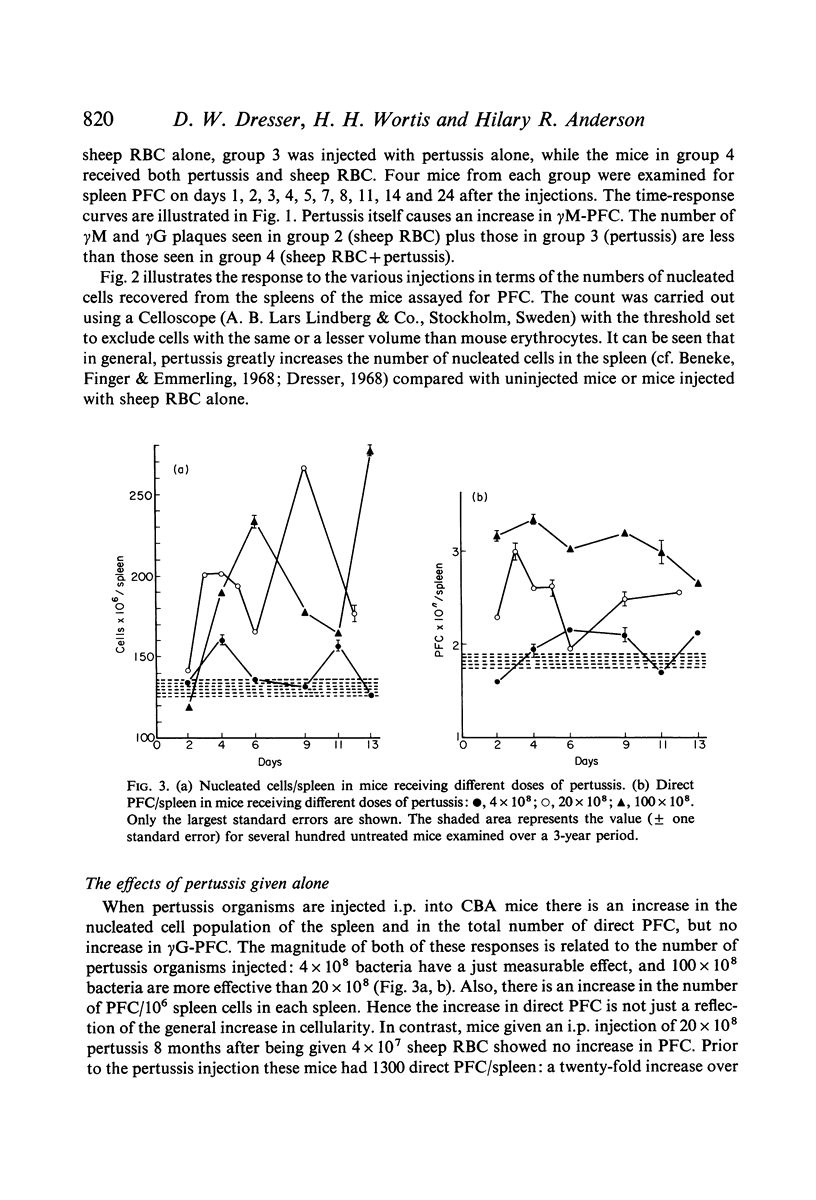
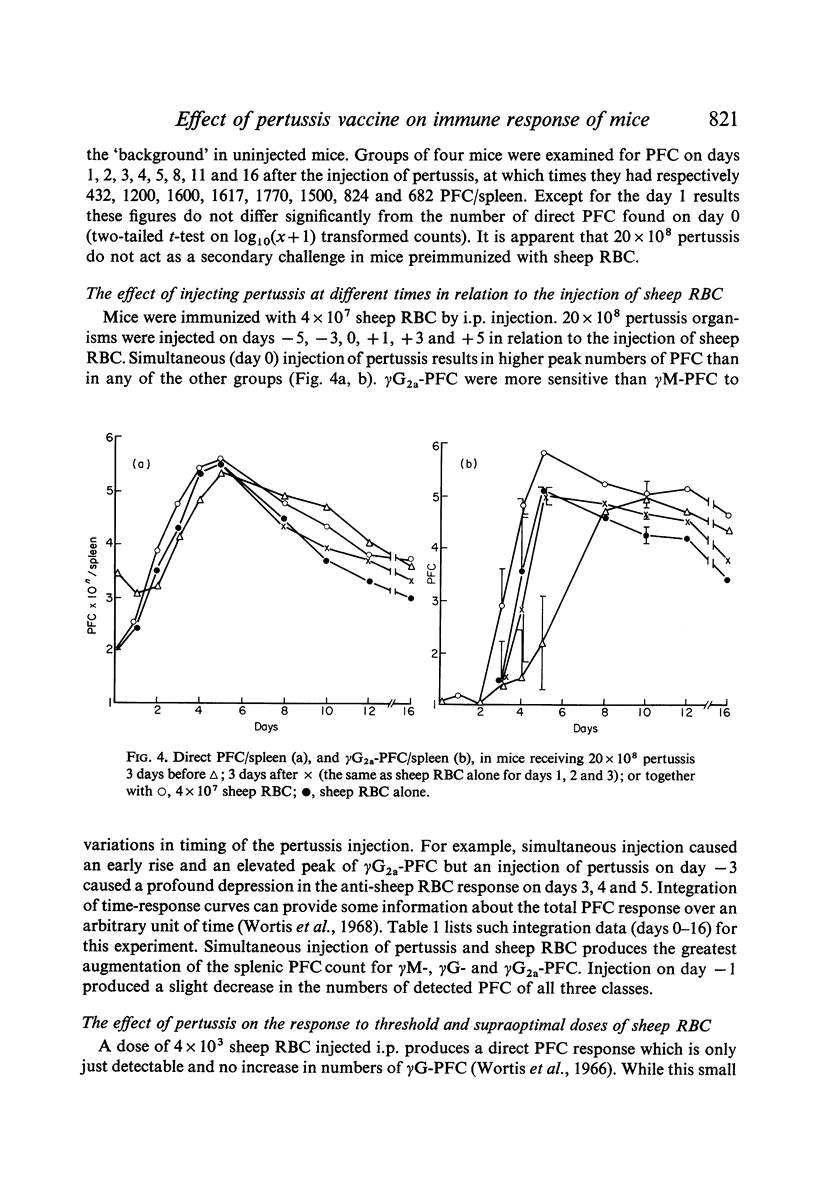
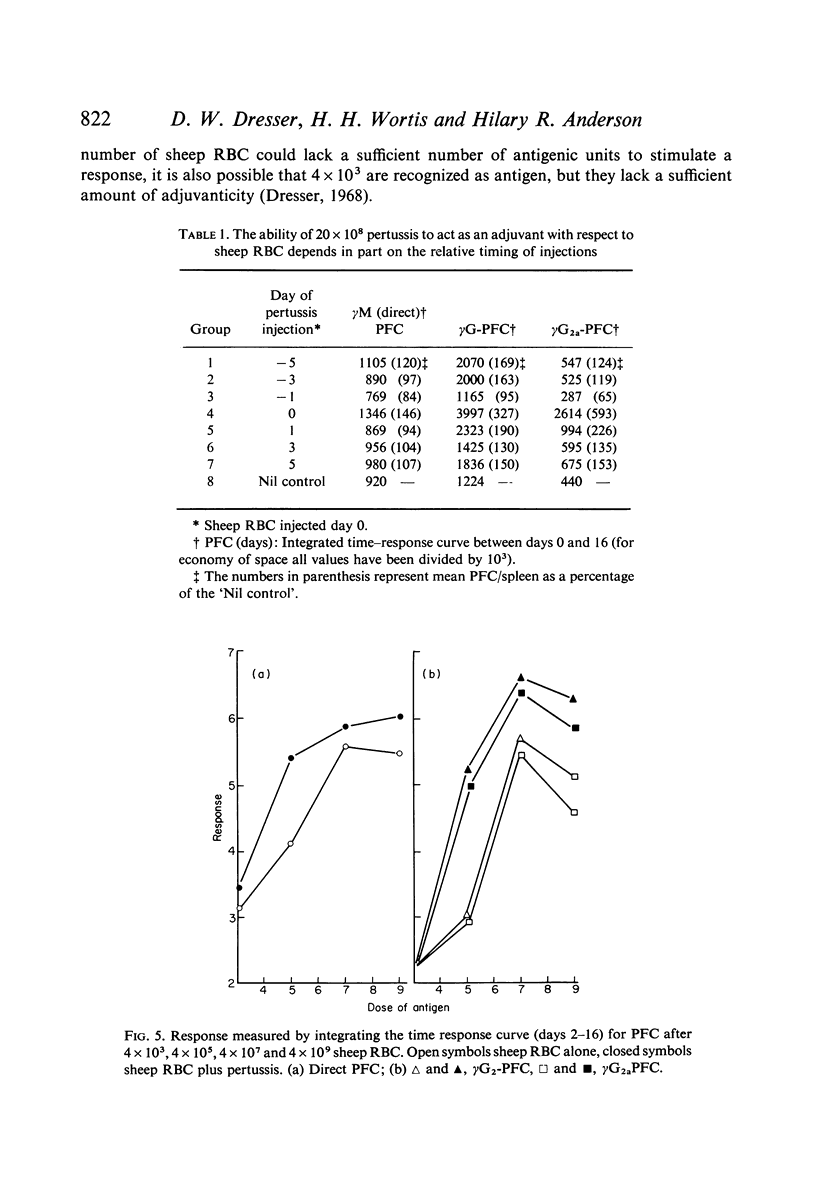
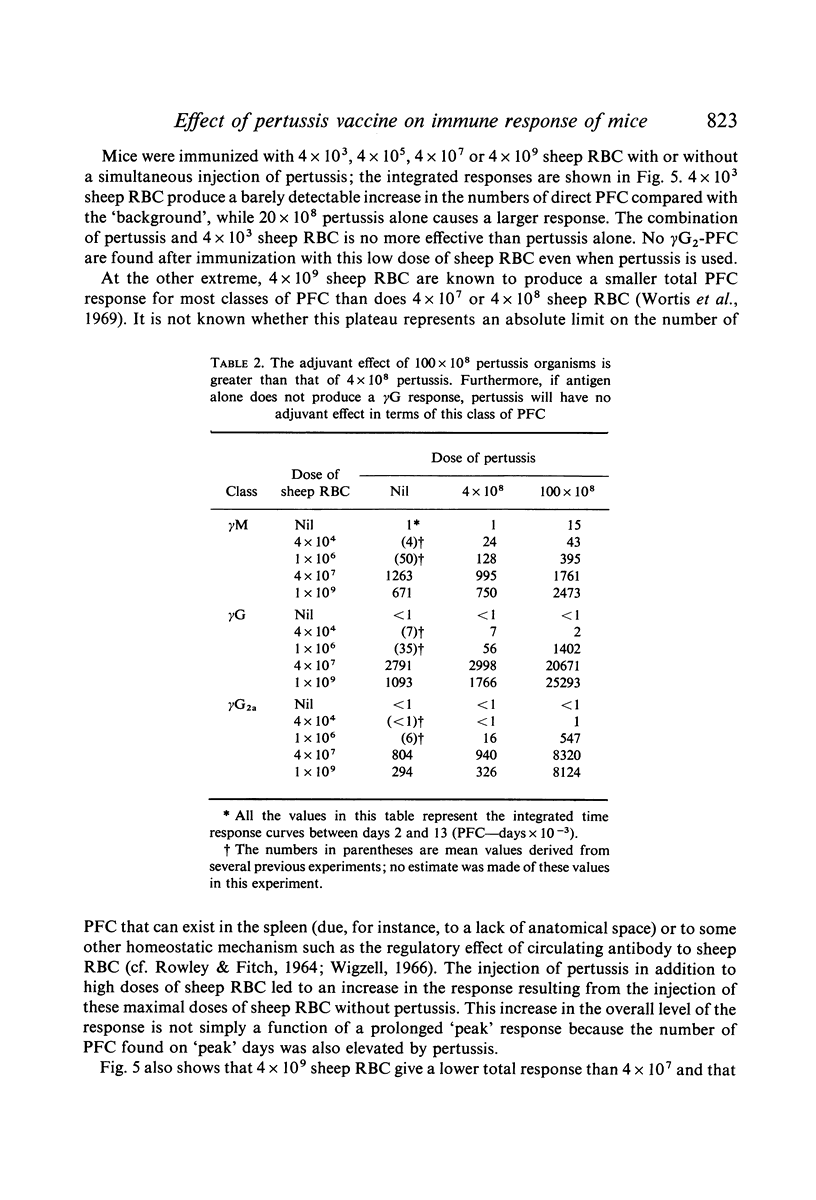
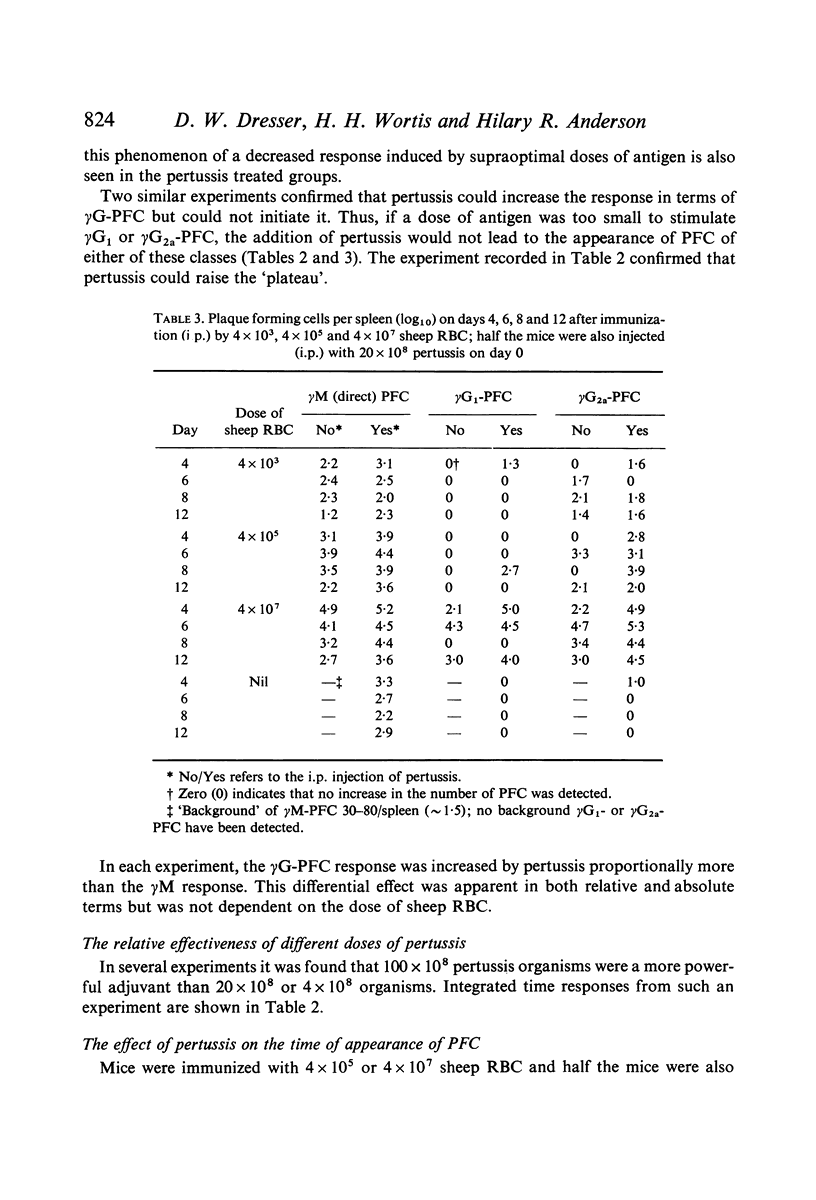
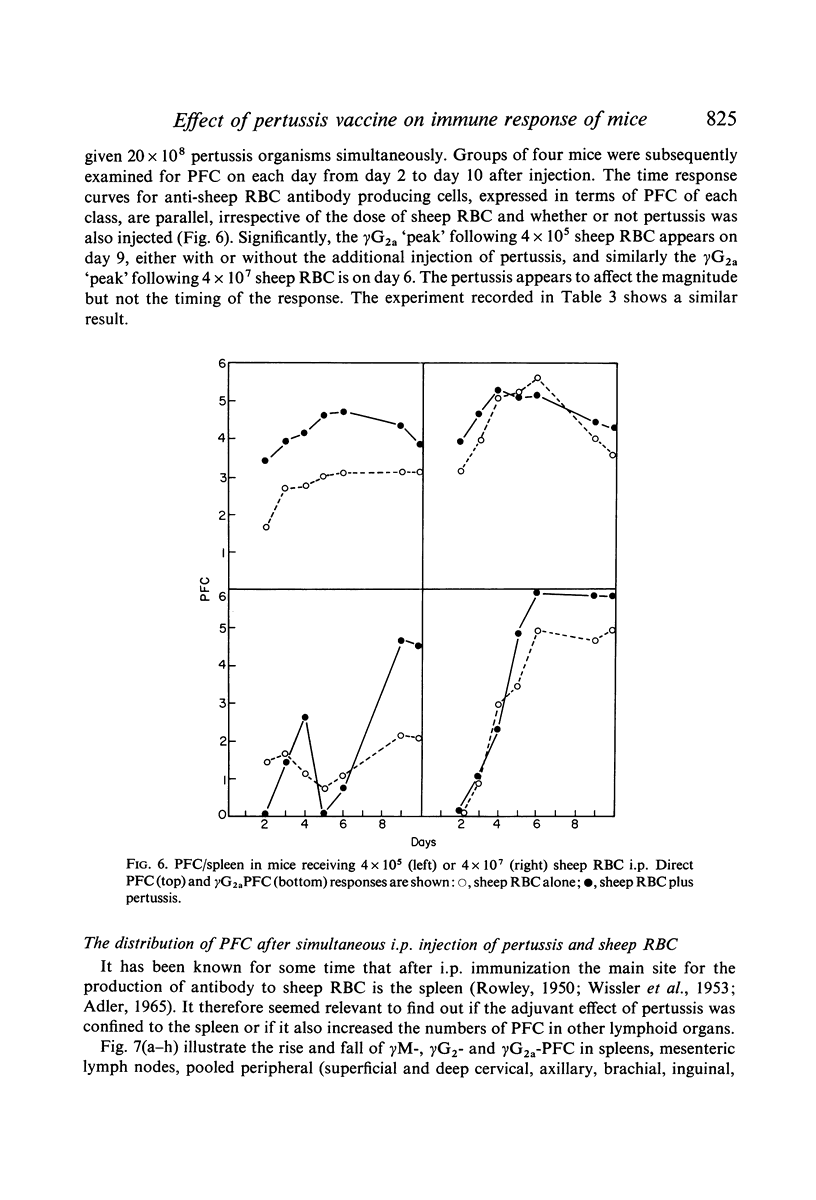
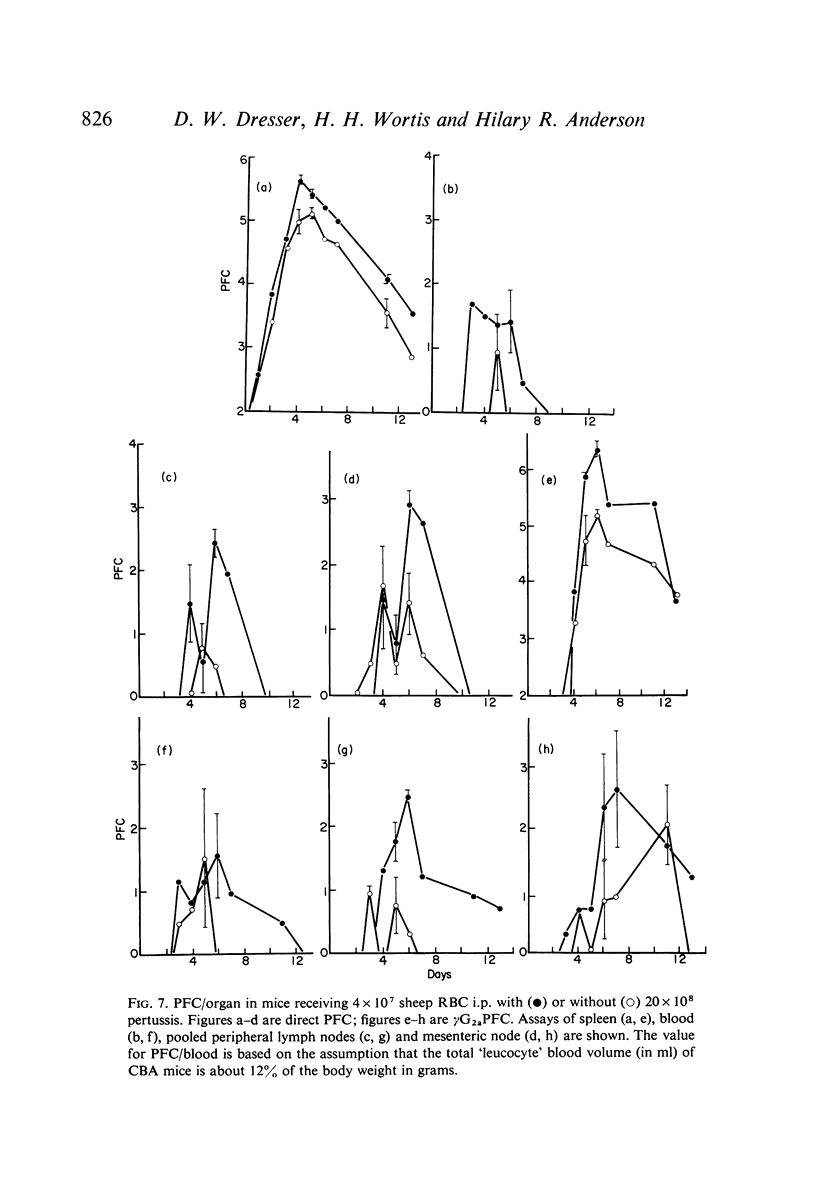
Bordetella pertussis is an adjuvant when given to mice immunized with sheep RBC. The adjuvant activity of pertussis is reflected in an increase in the number of cells producing antibody as measured by the localized haemolysis in gel technique. γG-PFC have a more marked response to pertussis than do γM-PFC, and in general γG-PFC are more sensitive than γM to variations in dose or injection schedule of pertussis organisms. Pertussis increases the response to doses of antigen which previously were considered to be maximal. The distribution of PFC between spleen, blood and lymph nodes is altered by pertussis injections.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER F. L. STUDIES ON MOUSE ANTIBODIES. I. THE RESPONSE TO SHEEP RED CELLS. J Immunol. 1965 Jul;95:26–38. [PubMed] [Google Scholar]
- Barth W. F., McLaughlin C. L., Fahey J. L. The immunoglobulins of mice. VI. Response to immunization. J Immunol. 1965 Nov;95(5):781–790. [PubMed] [Google Scholar]
- DRESSER D. W. Effectiveness of lipid and lipidophilic substances as adjuvants. Nature. 1961 Sep 16;191:1169–1171. doi: 10.1038/1911169a0. [DOI] [PubMed] [Google Scholar]
- Dresser D. W. An assay for adjuvanticity. Clin Exp Immunol. 1968 Nov;3(9):877–888. [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., WUNDERLICH J., MISHELL R. THE IMMUNOGLOBULINS OF MICE. II. TWO SUBCLASSES OF MOUSE 7S GAMMA-2-GLOBULINS: GAMMA-2A- AND GAMMA-2B-GLOBULINS. J Exp Med. 1964 Aug 1;120:243–251. doi: 10.1084/jem.120.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger H., Emmerling P., Schmidt H. Accelerated and prolongated multiplication of antibody-forming spleen cells by Bordetella pertussis in mice immunized with sheep red blood cells. Experientia. 1967 Jul 15;23(7):591–592. doi: 10.1007/BF02137991. [DOI] [PubMed] [Google Scholar]
- Finger H., Emmerling P., Tusch H., Bredt W. Einfluss von Bordetta pertussis auf das lymphatische Gewebe von Mäusen. 3. Die beeinflussung der Kinetik der Antikörperbildung gegen Schaferythrozyten durch Bordetella pertussis. Z Immunitatsforsch Allerg Klin Immunol. 1968 Aug-Sep;136(3):268–284. [PubMed] [Google Scholar]
- KIND L. S. Relationship of the anaphylaxis sensitizing and adjuvant properties of Hemophilus pertussis vaccine. J Immunol. 1957 Sep;79(3):238–242. [PubMed] [Google Scholar]
- Kalpaktsoglou P. K., Yunis E. J., Good R. A. Changes produced by pertussis antigen on the blood cells and lympho-haemapoietic tissues after early and late thymectomy or splenectomy. Clin Exp Immunol. 1969 Jul;5(1):91–103. [PMC free article] [PubMed] [Google Scholar]
- MORSE S. I. STUDIES ON THE LYMPHOCYTOSIS INDUCED IN MICE BY BORDETELLA PERTUSSIS. J Exp Med. 1965 Jan 1;121:49–68. doi: 10.1084/jem.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):821–837. doi: 10.1084/jem.128.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. I., Riester S. K. Studies on the leukocytosis and lymphocytosis induced by Bordetella pertussis. II. The effect of pertussis vaccine on the thoracic duct lymph and lymphocytes of mice. J Exp Med. 1967 Apr 1;125(4):619–628. doi: 10.1084/jem.125.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. A., FITCH F. W. HOMEOSTASIS OF ANTIBODY FORMATION IN THE ADULT RAT. J Exp Med. 1964 Dec 1;120:987–1005. doi: 10.1084/jem.120.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D. A. The effect of splenectomy on the formation of circulating antibody in the adult male albino rat. J Immunol. 1950 Apr;64(4):289–295. [PubMed] [Google Scholar]
- Taub R. N., Krantz A. R., Dresser D. W. The effect of localized injection of adjuvant material on the draining lymph node. I. Histology. Immunology. 1970 Feb;18(2):171–186. [PMC free article] [PubMed] [Google Scholar]
- WISSLER R. W., ROBSON M. J., FITCH F., NELSON W., JACOBSON L. O. The effects of spleen shielding and subsequent splenectomy upon antibody formation in rats receiving total-body x-irradiation. J Immunol. 1953 Apr;70(4):379–385. [PubMed] [Google Scholar]
- Wigzell H. Antibody synthesis at the cellular level. Antibody-induced suppression of 7S antibody synthesis. J Exp Med. 1966 Nov 1;124(5):953–969. doi: 10.1084/jem.124.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C., Fleming W. A., White R. G. The effect of adjuvants on biosynthesis of 19S and 7S antibody against bacteriophage phiX174 in the guinea-pig. Immunology. 1967 Dec;13(6):603–611. [PMC free article] [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the LHG assay. I. The splenic response of CBA mice to sheep erythrocytes. Immunology. 1966 Dec;11(6):603–616. [PMC free article] [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the localized haemolysis in gel (LHG) assay. II. Assay procedure. Immunology. 1968 Jan;14(1):69–79. [PMC free article] [PubMed] [Google Scholar]


