Abstract
Antithymus globulin (ATG) and antibursa globulin (ABG) prepared in rabbits with thymus and bursa cells were used in experiments on New Hampshire embryos. The in vitro assay of potency of ATG and ABG by means of absorption, leucoagglutination, cytotoxicity test and passive haemagglutination showed that ATG and ABG react equally well with both thymus and bursa antigens.
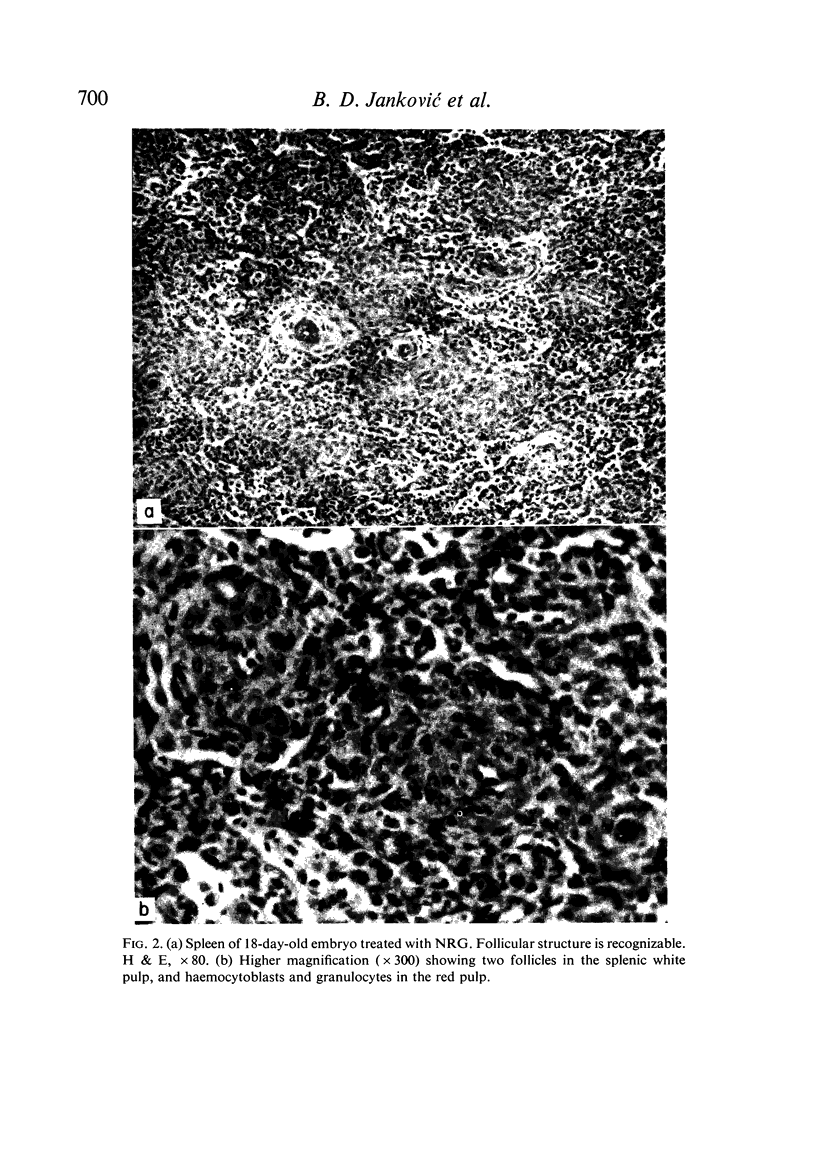
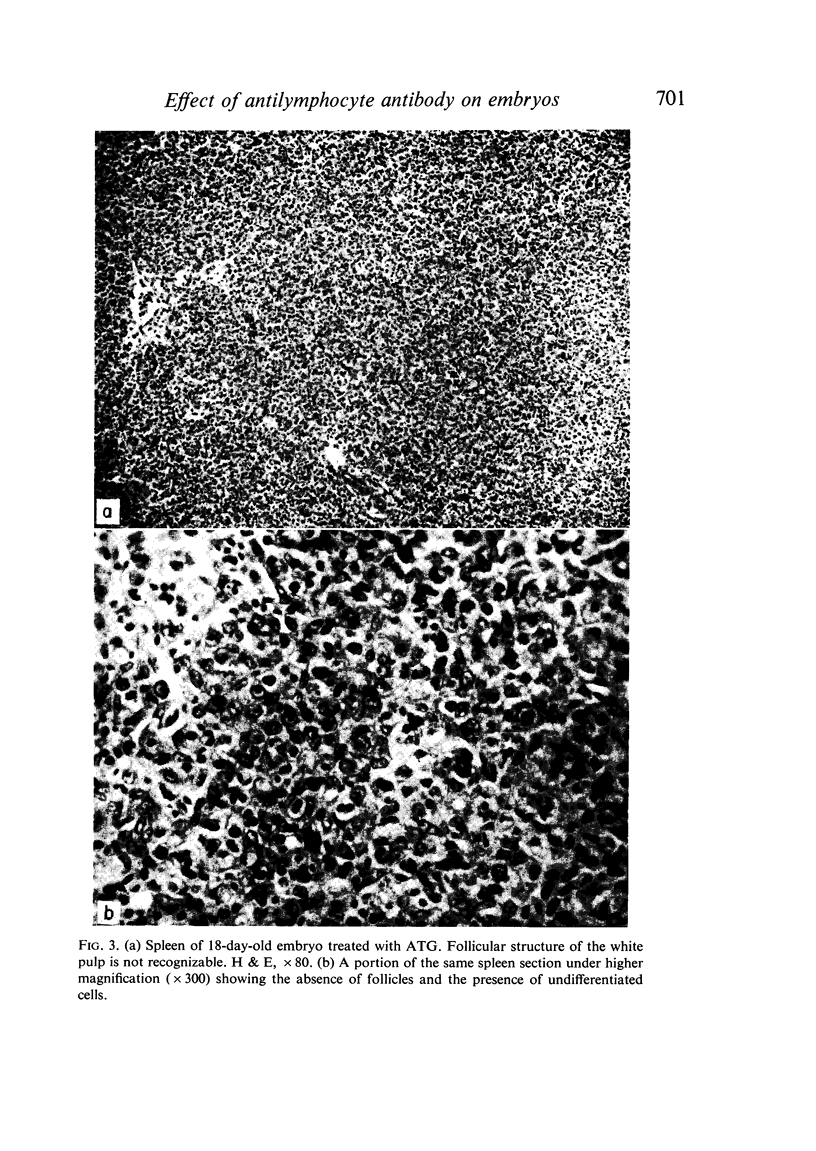
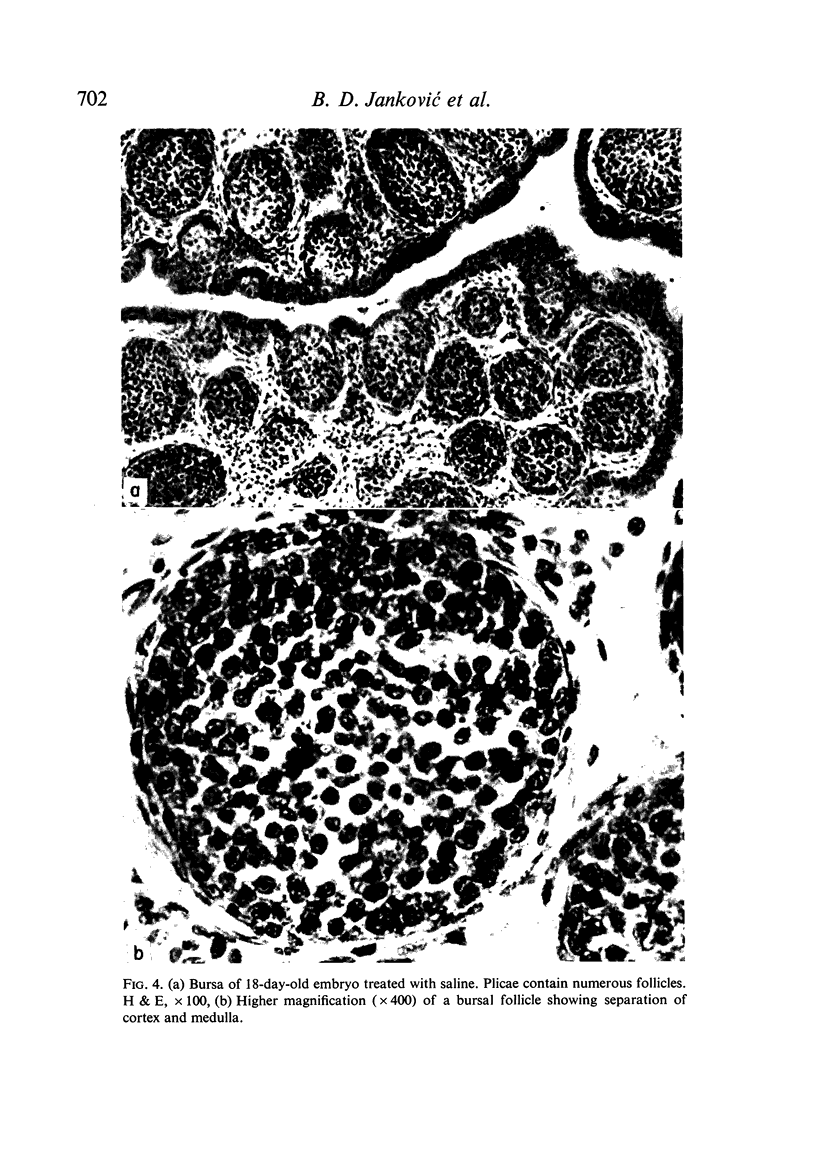
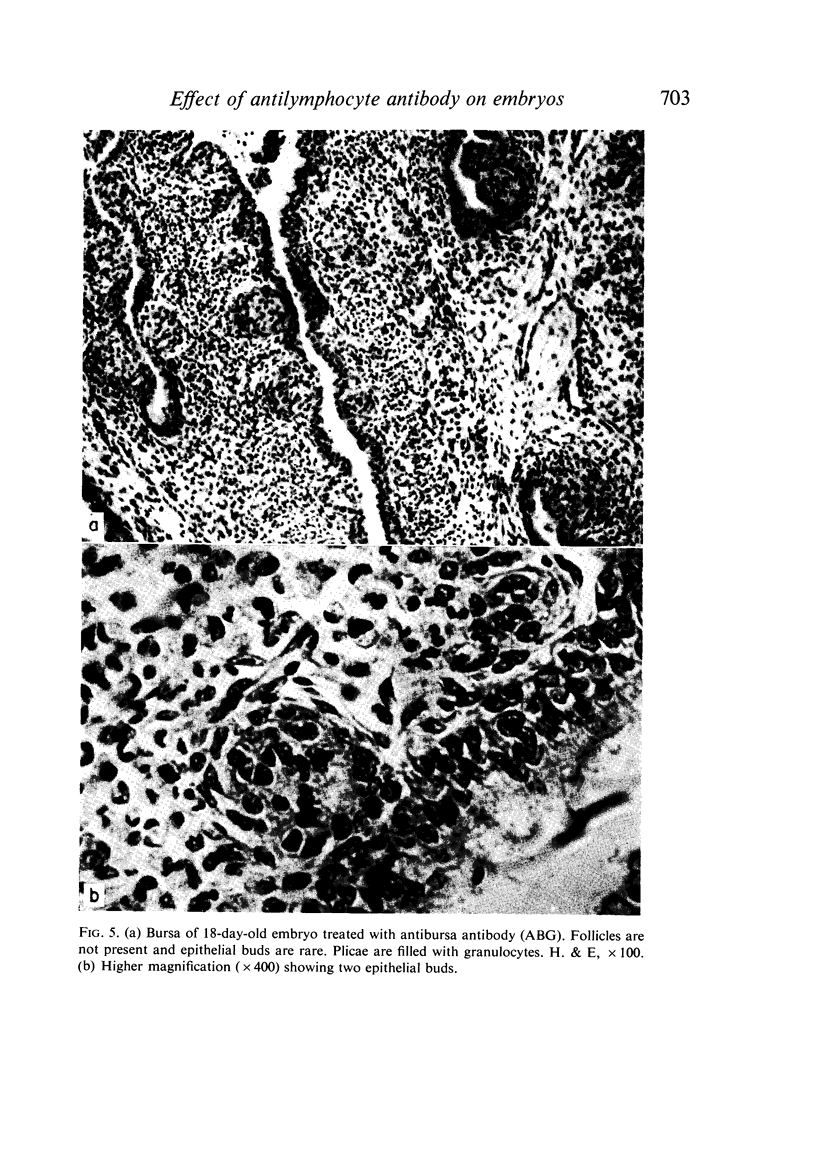
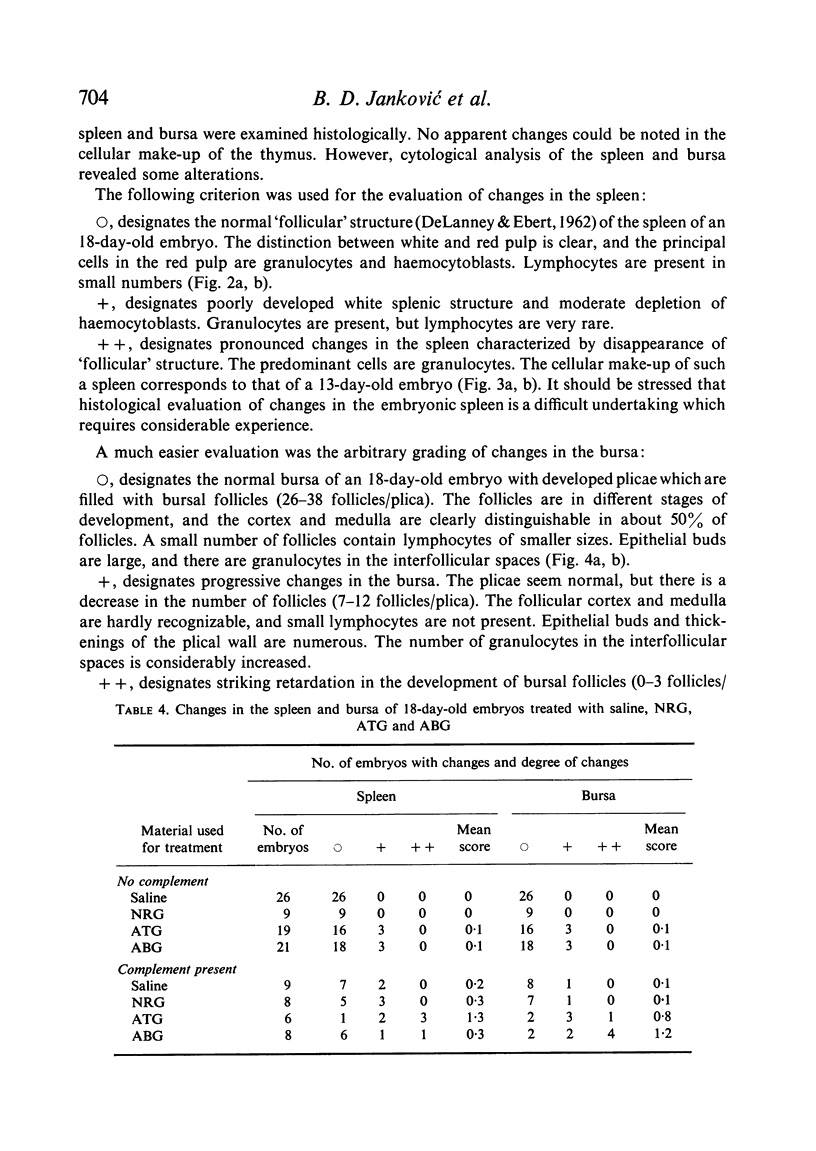
Seven-day-old embryos were treated with eleven intrachorioallantoic injections of ATG, ABG and NRG (normal rabbit globulin) from the seventh to seventeenth day of incubation. The incidence of death in embryos receiving ATG and ABG was very high when compared with controls injected with NRG and saline. The ATG affected the cellular make-up of the spleen and bursa, while ABG influenced only the bursa. The cytotoxic-like and development-retarding activity of ATG and ABG were possible only in the presence of guinea-pig complement. The embryonic thymus proved to be resistant to the action of heterologous antilymphocyte antibody.
Full text
PDF



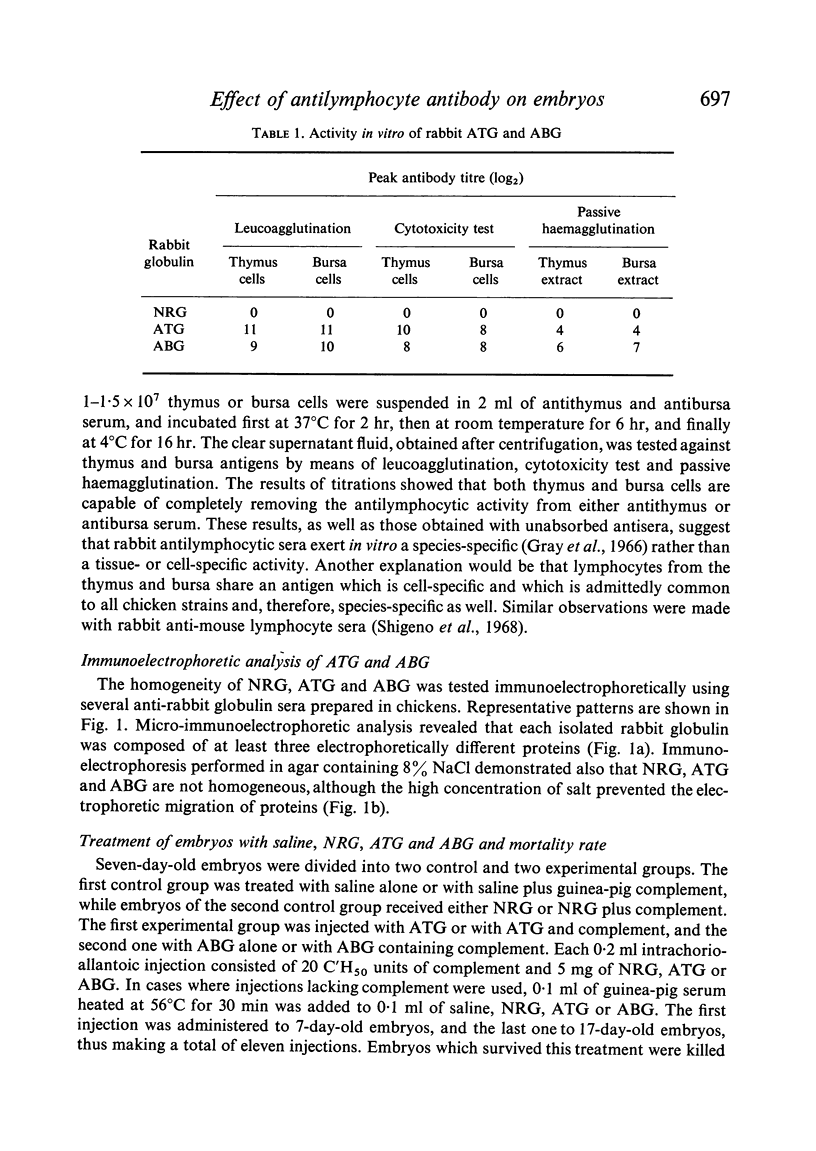



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLANTYNE D. L., Jr Modifications of technique used for tissue-grafting to the chorioallantoic membrane of the chick embryo. Transplant Bull. 1959 Jan;6(1):110–113. doi: 10.1097/00006534-195901000-00044. [DOI] [PubMed] [Google Scholar]
- Denman A. M. Anti-lymphocytic antibody and autoimmune disease: a review. Clin Exp Immunol. 1969 Sep;5(3):217–249. [PMC free article] [PubMed] [Google Scholar]
- Field E. O., Gibbs J. E. Cross-reaction of anti-lymphocyte serum with haemopoietic stem cells. Nature. 1968 Feb 10;217(5128):561–562. doi: 10.1038/217561a0. [DOI] [PubMed] [Google Scholar]
- Gray J. G., Monaco A. P., Wood M. L., Russell P. S. Studies on heterologous anti-lymphocyte serum in mice. I. In vitro and in vivo properties. J Immunol. 1966 Feb;96(2):217–228. [PubMed] [Google Scholar]
- JANKOVIC B. D., ISAKOVIC K., MIHAILOVIC L. Organ-specificity of lipids extracted from various regions of cat brain. Int Arch Allergy Appl Immunol. 1960;17:211–220. [PubMed] [Google Scholar]
- James K. Anti-lymphocytic antibody--a review. Clin Exp Immunol. 1967 Nov;2(6):615–631. [PMC free article] [PubMed] [Google Scholar]
- MIHAILOVIC L., JANKOVIC B. D. Effects of intraventricularly injected anti-n. caudatus antibody on the electrical activity of the cat brain. Nature. 1961 Nov 18;192:665–666. doi: 10.1038/192665a0. [DOI] [PubMed] [Google Scholar]
- NACE G. W. Development in the presence of antibodies. Ann N Y Acad Sci. 1955 Jun 2;60(7):1038–1055. doi: 10.1111/j.1749-6632.1955.tb40088.x. [DOI] [PubMed] [Google Scholar]
- ROSE M. E., ORLANS E. Fowl antibody: III. Its haemolytic activity with complements of various species and some properties of fowl complement. Immunology. 1962 Nov;5:633–641. [PMC free article] [PubMed] [Google Scholar]
- Russell P. S. Antilymphocyte serum as an immunosuppressive agent. Ann Intern Med. 1968 Feb;68(2):483–486. doi: 10.7326/0003-4819-68-2-483. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Shigeno N., Hämmerling U., Arpels C., Boyse E. A., Old L. J. Preparation of lymphocyte-specific antibody from anti-lymphocyte serum. Lancet. 1968 Aug 10;2(7563):320–323. doi: 10.1016/s0140-6736(68)90530-8. [DOI] [PubMed] [Google Scholar]
- Tempelis C. H., Lofy M. F. An adaptation of the immunoelectrophoretic method for the use of chicken-precipitating antisera. J Immunol. 1965 Sep;95(3):418–421. [PubMed] [Google Scholar]