Abstract
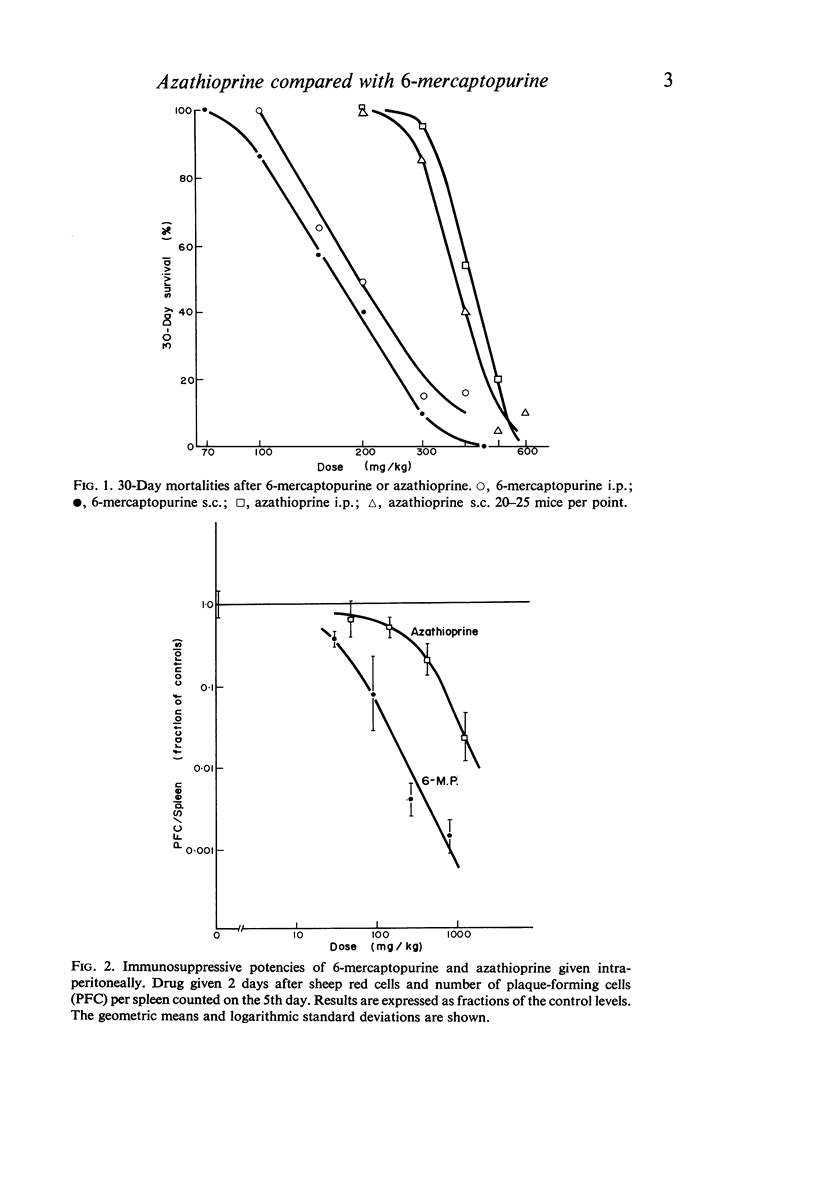
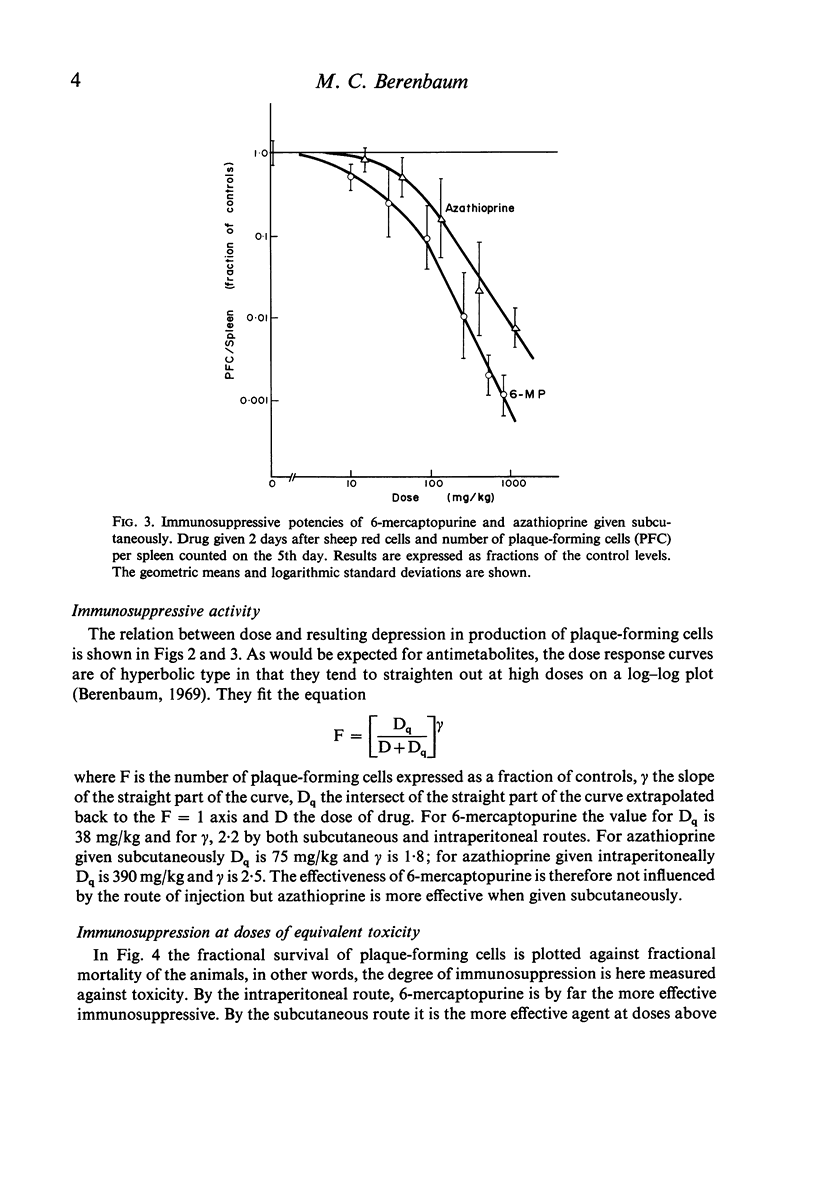
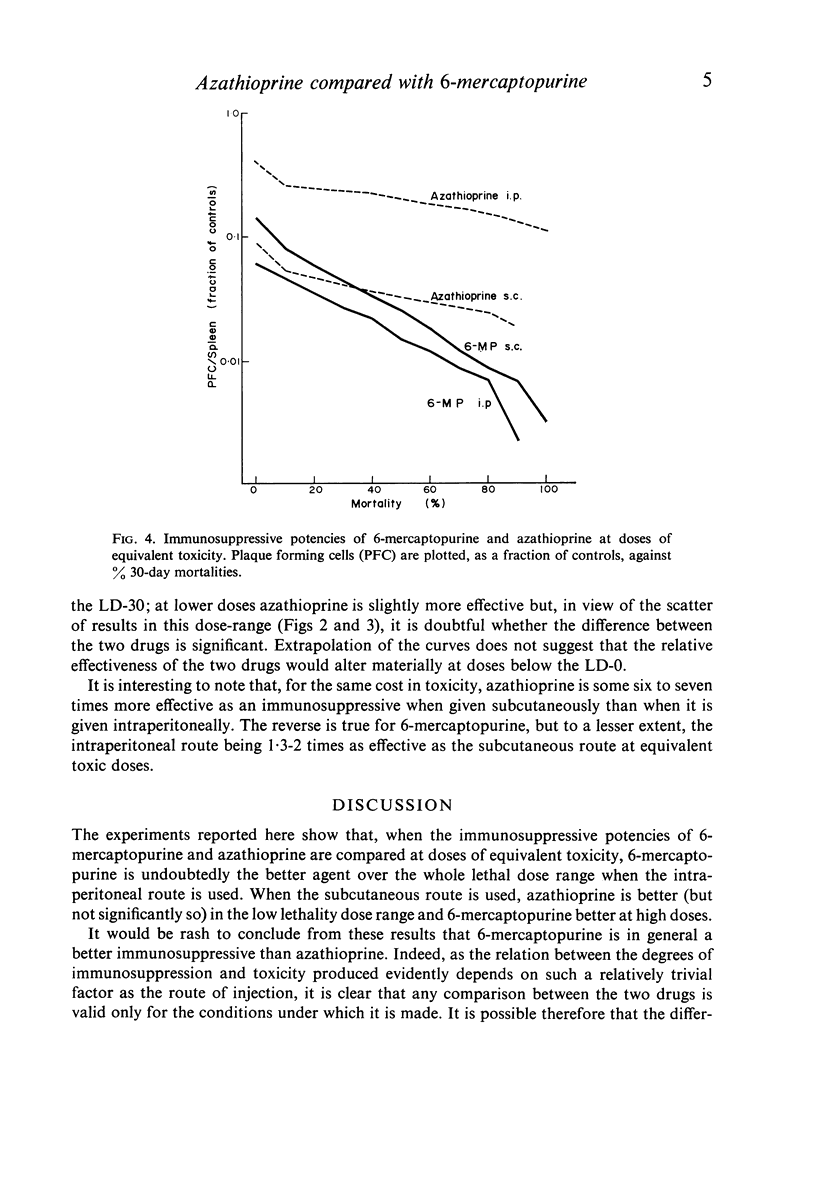
The toxicities and immunosuppressive potencies of single doses of 6-mercaptopurine and azathioprine have been compared in mice, using the 30-day mortality as a measure of toxicity, and reduction in spleen plaque-forming cells in response to sheep erythrocytes as a measure of immunosuppression. When compared on the basis of equivalent toxicity, 6-mercaptopurine was consistently the more effective agent by the intraperitoneal route. By the subcutaneous route, 6-mercaptopurine was more effective at doses above the LD-30; at lower doses, azathioprine was marginally better, but the difference was probably not significant. For the same cost in toxicity, azathioprine was six to seven times more effective as an immunosuppressive by the subcutaneous as by the intraperitoneal route.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERENBAUM M. C., BROWN I. N. DOSE-RESPONSE RELATIONSHIPS FOR AGENTS INHIBITING THE IMMUNE RESPONSE. Immunology. 1964 Jan;7:65–71. [PMC free article] [PubMed] [Google Scholar]
- Berenbaum M. C. Dose-response curves for agents that impair cell reproductive integrity. A fundamental difference between dose-response curves of antimetabolites and those for radiation and alkylating agents. Br J Cancer. 1969 Jun;23(2):426–433. doi: 10.1038/bjc.1969.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALNE R. Y. The rejection of renal homografts. Inhibition in dogs by 6-mercaptopurine. Lancet. 1960 Feb 20;1(7121):417–418. doi: 10.1016/s0140-6736(60)90343-3. [DOI] [PubMed] [Google Scholar]
- DAMESHEK W., SCHWARTZ R. Treatment of certain "autoimmune" diseases with antimetabolites; a preliminary report. Trans Assoc Am Physicians. 1960;73:113–127. [PubMed] [Google Scholar]
- FRISCH A. W., DAVIES G. H. The inhibition of hemagglutinin formation in mice by purine and pyrimidine analogues. J Immunol. 1962 Mar;88:269–273. [PubMed] [Google Scholar]
- GOODMAN H. C., WOLFF S. M., CARPENTER R. R., ANDERSEN B. R., BRANDRISS M. W. CURRENT STUDIES ON THE EFFECT ANTIMETABOLITES IN NEPHROSIS, OTHER NON-NEOPLASTIC DISEASES, AND EXPERIMENTAL ANIMALS. COMBINED CLINICAL STAFF CONFERENCE AT THE NATIONAL INSTITUTES OF HEALTH. Ann Intern Med. 1963 Sep;59:388–407. doi: 10.7326/0003-4819-59-3-388. [DOI] [PubMed] [Google Scholar]
- Hume D. M., Lee H. M., Williams G. M., White H. J., Ferré J., Wolf J. S., Prout G. R., Jr, Slapak M., O'Brien J., Kilpatrick S. J. Comparative results of cadaver and related donor renal homografts in man, and immunologic implications of the outcome of second and paired transplants. Ann Surg. 1966 Sep;164(3):352–397. doi: 10.1097/00000658-196609000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- NATHAN H. C., BIEBER S., ELION G. B., HITCHINGS G. H. Detection of agents which interfere with the immune response. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:796–799. doi: 10.3181/00379727-107-26758. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ R., STACK J., DAMESHEK W. Effect of 6-mercaptopurine on antibody production. Proc Soc Exp Biol Med. 1958 Oct;99(1):164–167. doi: 10.3181/00379727-99-24281. [DOI] [PubMed] [Google Scholar]



