Abstract
Evidence is presented, based on in vitro model-systems, that lymphokine-like agents and lymphocytes can elicit in vitro changes in macrophages which replicate those observed in vivo associated with cell-mediated immunity.
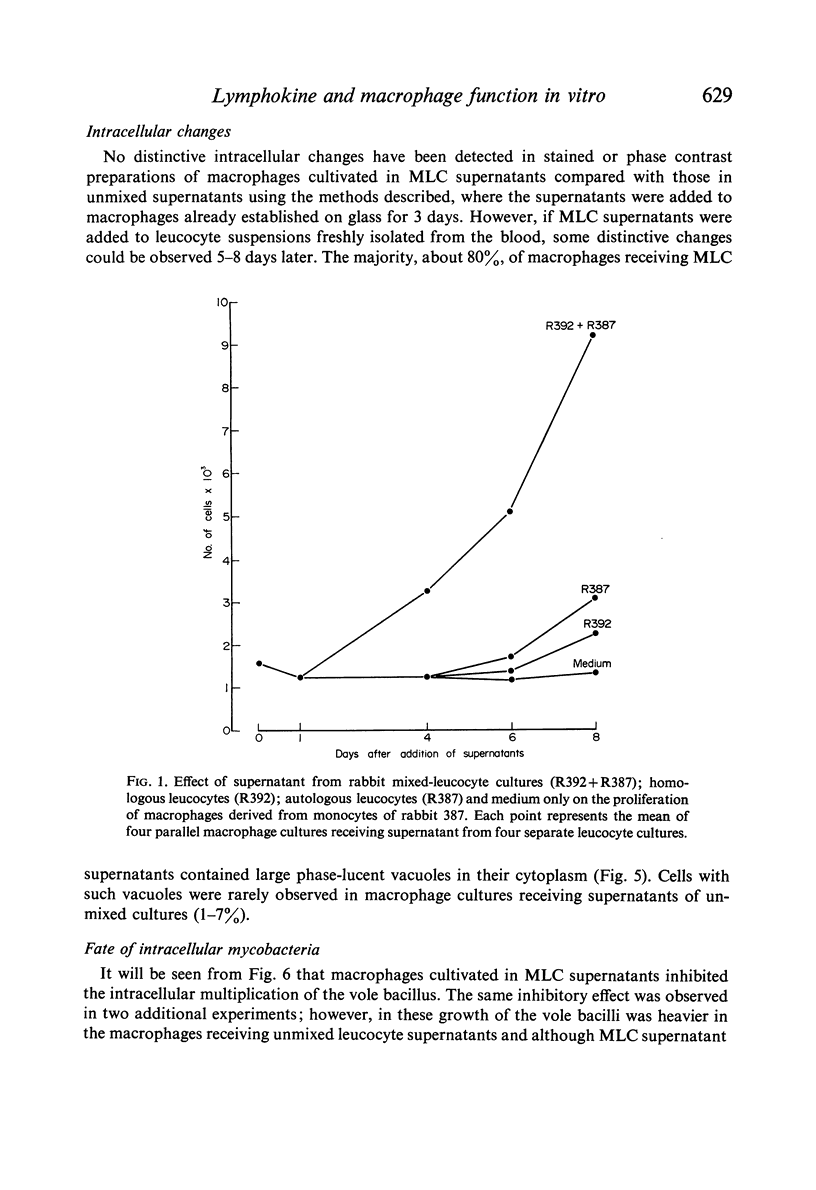
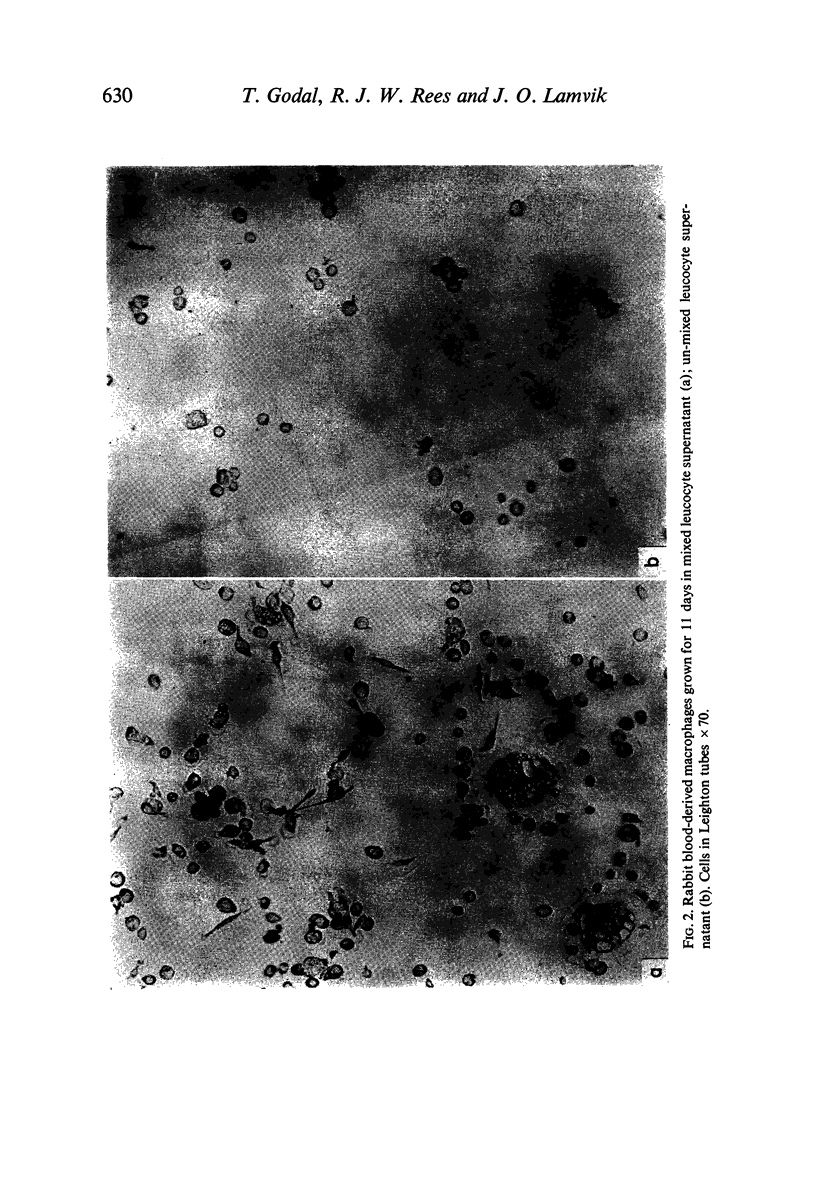
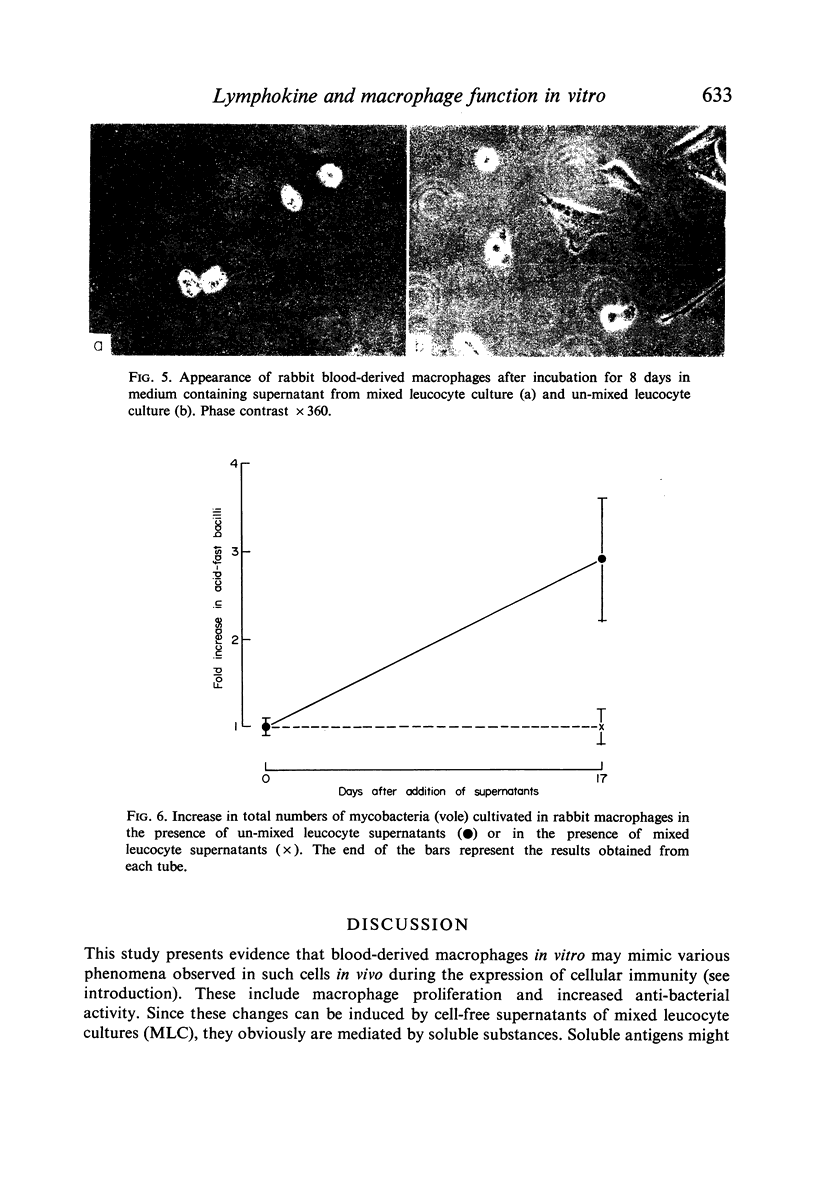
(1) Supernatants (containing lymphokines; filtered or unfiltered) from mixed leucocyte cultures (MLC) from two genetically different rabbits activated rabbit blood-derived macrophages cultured in vitro. Activation comprised proliferation, presence of intracellular phase-lucent vacuoles, elongation and formation of intercellular cytoplasmic bridges and giant cells. No such activation was obtained with supernatants from unmixed leucocyte cultures.
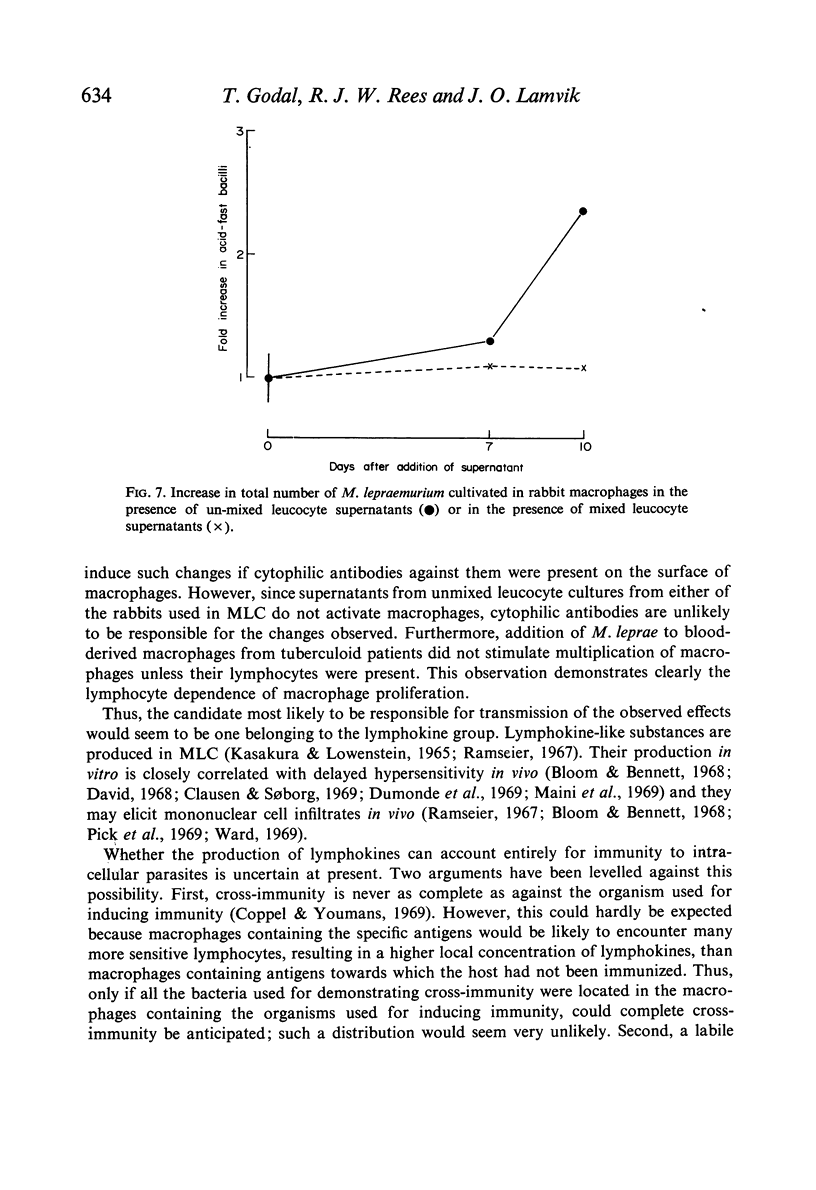
(2) Macrophages cultivated in MLC supernatants, but not unmixed leucocyte supernatants, inhibited the intracellular multiplication of mycobacteria, including the vole bacillus and Mycobacterium lepraemurium.
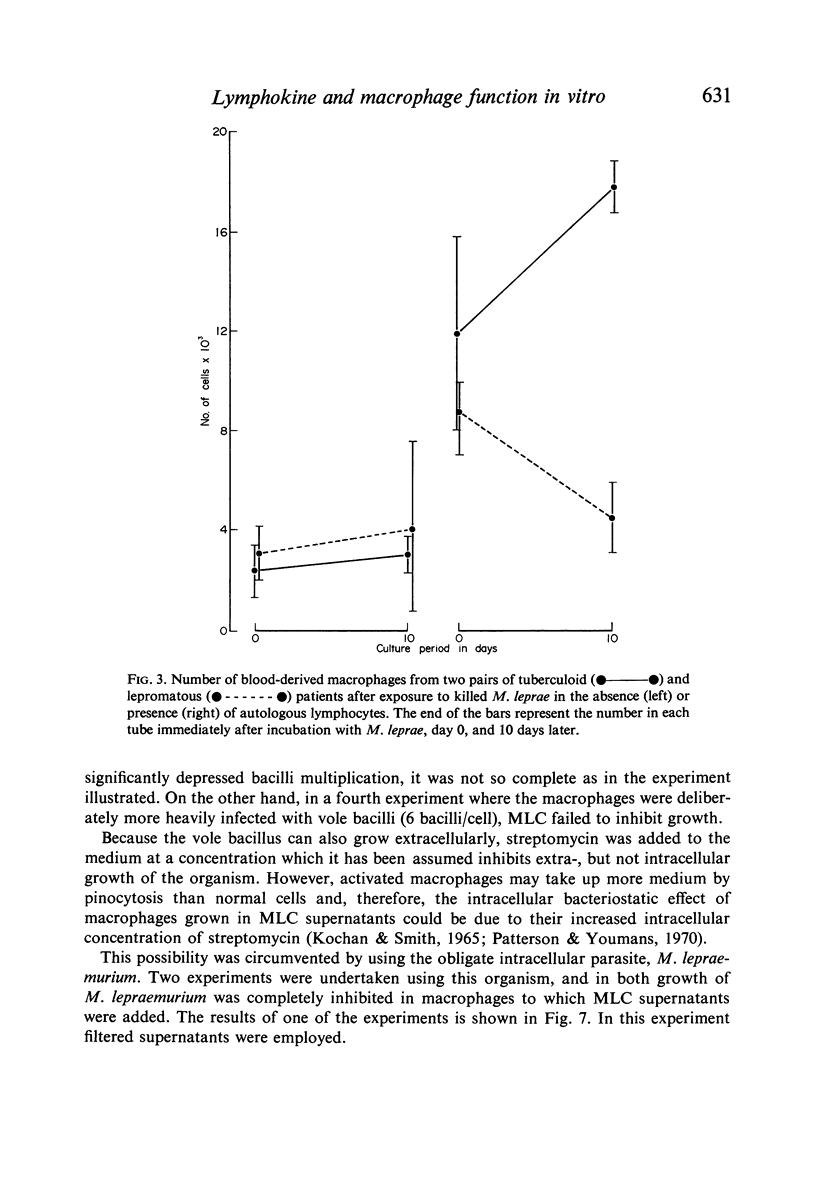
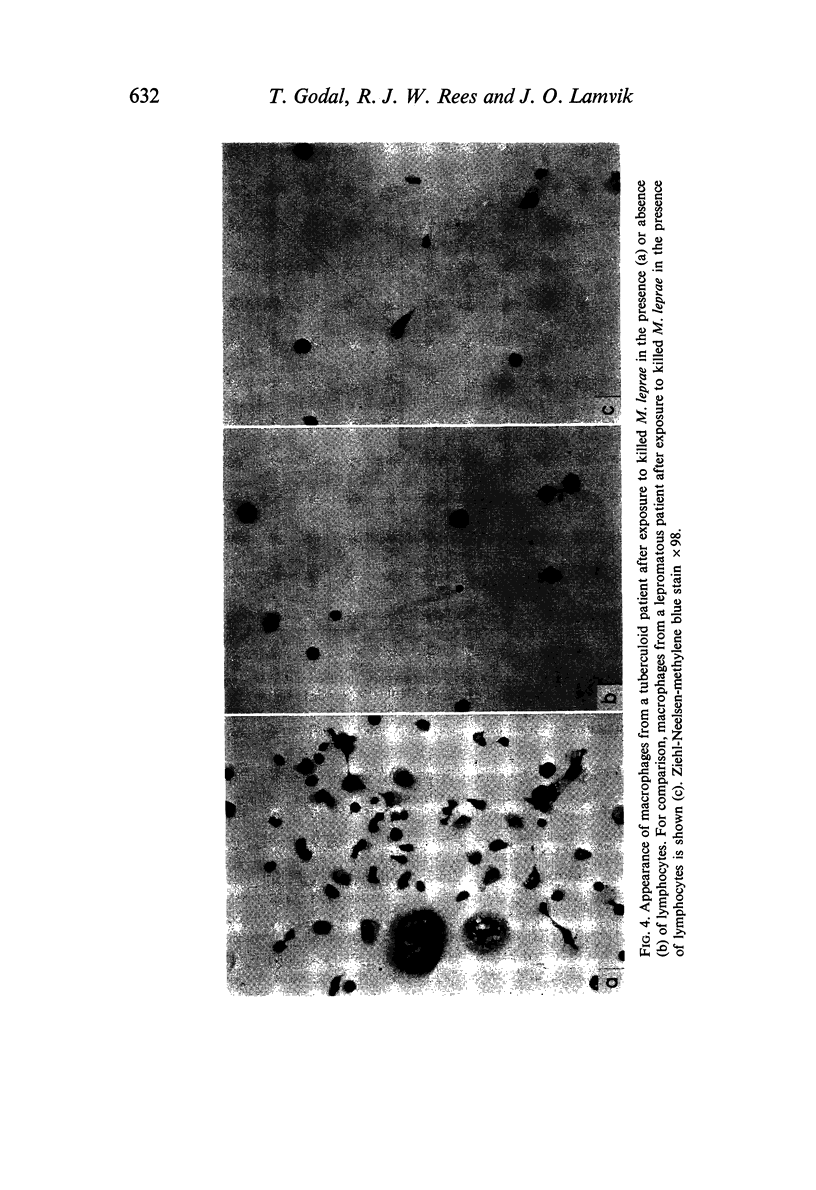
(3) Similar macrophage activation was obtained in cultures of blood-derived macrophages exposed to M. leprae in vitro from patients with tuberculoid leprosy (high resistant form) in the presence of lymphocytes. No such activation was obtained in absence of lymphocytes. Under similar conditions no activation was observed in cultures of macrophages from patients with lepromatous leprosy (low resistant form of the disease).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Hart P. D. Potentiation by silica of the growth of Mycobacterium tuberculosis in macrophage cultures. Br J Exp Pathol. 1968 Oct;49(5):465–476. [PMC free article] [PubMed] [Google Scholar]
- BAIN B., VAS M. R., LOWENSTEIN L. THE DEVELOPMENT OF LARGE IMMATURE MONONUCLEAR CELLS IN MIXED LEUKOCYTE CULTURES. Blood. 1964 Jan;23:108–116. [PubMed] [Google Scholar]
- BERTHRONG M., HAMILTON M. A. Tissue culture studies on resistance in tuberculosis. II. Monocytes from normal and immunized guinea pigs infected with virulent human tubercle bacilli. Am Rev Tuberc. 1959 Feb;79(2):221–231. doi: 10.1164/artpd.1959.79.2.221. [DOI] [PubMed] [Google Scholar]
- Bennett W. E., Cohn Z. A. The isolation and selected properties of blood monocytes. J Exp Med. 1966 Jan 1;123(1):145–160. doi: 10.1084/jem.123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Migration inhibitory factor associated with delayed-type hypersensitivity. Fed Proc. 1968 Jan-Feb;27(1):13–15. [PubMed] [Google Scholar]
- Boak J. L., Kölsch E., Mitchison N. A. Immunological tolerance and inhibition by hapten. Antibiot Chemother. 1969;15:98–109. doi: 10.1159/000386774. [DOI] [PubMed] [Google Scholar]
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- Clausen J. E., Soborg M. In vitro detection of tuberculin hypersensitivity in man. Specific migration inhibition of white blood cells from tuberculin-positive persons. Acta Med Scand. 1969 Sep;186(3):227–230. [PubMed] [Google Scholar]
- Coppel S., Youmans G. P. Specificity of the anamnestic response produced by Listeria monocytogenes or Mycobacterium tuberculosis to challenge with Listeria monocytogenes. J Bacteriol. 1969 Jan;97(1):127–133. doi: 10.1128/jb.97.1.127-133.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. R. Macrophage migration. Fed Proc. 1968 Jan-Feb;27(1):6–12. [PubMed] [Google Scholar]
- Dumonde D. C., Wolstencroft R. A., Panayi G. S., Matthew M., Morley J., Howson W. T. "Lymphokines": non-antibody mediators of cellular immunity generated by lymphocyte activation. Nature. 1969 Oct 4;224(5214):38–42. doi: 10.1038/224038a0. [DOI] [PubMed] [Google Scholar]
- Fildes C., Hart P. D. Use of a detergent-acid mixture to rupture tissue culture cells. Nature. 1967 May 6;214(5088):624–625. doi: 10.1038/214624a0. [DOI] [PubMed] [Google Scholar]
- GARBUTT E. W., REES R. J., BARR Y. M. Growth of Mycobacterium lepraemurium maintained in cultures of rat fibrolasts. J Gen Microbiol. 1962 Feb;27:259–268. doi: 10.1099/00221287-27-2-259. [DOI] [PubMed] [Google Scholar]
- HART P. D., REES R. J. Effect of macrocyclon in acute and chronic pulmonary tuberculous infection in mice as shown by viable and total bacterial counts. Br J Exp Pathol. 1960 Aug;41:414–421. [PMC free article] [PubMed] [Google Scholar]
- KOCHAN I., SMITH L. ANTIMYCOBACTERIAL ACTIVITY OF TUBERCULOSTATIC FACTOR ON INTRACELLULAR BACILLI. J Immunol. 1965 Feb;94:220–227. [PubMed] [Google Scholar]
- Kasakura S., Lowenstein L. A factor stimulating DNA synthesis derived from the medium of leukocyte cultures. Nature. 1965 Nov 20;208(5012):794–795. doi: 10.1038/208794a0. [DOI] [PubMed] [Google Scholar]
- Lamvik J. O. A micro-incubation chamber technique for testing the immunological activity of cultured lymphocytes. Scand J Haematol. 1968;5(4):278–286. doi: 10.1111/j.1600-0609.1968.tb01749.x. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. The growth of tubercle bacilli in monocytes from normal and vaccinated rabbits. Am Rev Tuberc. 1954 Apr;69(4):495–504. doi: 10.1164/art.1954.69.4.495. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Mackaness G. B. The immunology of antituberculous immunity. Am Rev Respir Dis. 1968 Mar;97(3):337–344. doi: 10.1164/arrd.1968.97.3.337. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini R. N., Bryceson A. D., Wolstencroft R. A., Dumonde D. C. Lymphocyte mitogenic factor in man. Nature. 1969 Oct 4;224(5214):43–44. doi: 10.1038/224043a0. [DOI] [PubMed] [Google Scholar]
- North R. J. Cellular kinetics associated with the development of acquired cellular resistance. J Exp Med. 1969 Aug 1;130(2):299–314. doi: 10.1084/jem.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The mitotic potential of fixed phagocytes in the liver as revealed during the development of cellular immunity. J Exp Med. 1969 Aug 1;130(2):315–326. doi: 10.1084/jem.130.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Multiplication of Mycobacterium tuberculosis Within Normal and "Immune" Mouse Macrophages Cultivated With and Without Streptomycin. Infect Immun. 1970 Jan;1(1):30–40. doi: 10.1128/iai.1.1.30-40.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Krejci J., Cech K., Turk J. L. Interaction between 'sensitized lymphocytes' and antigen in vitro. I. The release of a skin reactive factor. Immunology. 1969 Nov;17(5):741–767. [PMC free article] [PubMed] [Google Scholar]
- Ramseier H. Leukotactic factor elaborated by mixtures of genetically dissimilar cells. Science. 1967 Aug 4;157(3788):554–556. doi: 10.1126/science.157.3788.554. [DOI] [PubMed] [Google Scholar]
- SUTER E. Multiplication of tubercle bacilli within mononuclear phagocytes in tissue cultures derived from normal animals and animals vaccinated with BCG. J Exp Med. 1953 Feb 1;97(2):235–245. doi: 10.1084/jem.97.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector W. G., Walters M. N., Willoughby D. A. The origin of the mononuclear cells in inflammatory exudates induced by fibrinogen. J Pathol Bacteriol. 1965 Jul;90(1):181–192. doi: 10.1002/path.1700900119. [DOI] [PubMed] [Google Scholar]
- Turk J. L. Cell-mediated immunological processes in leprosy. Bull World Health Organ. 1969;41(6):779–792. doi: 10.5935/0305-7518.19700030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLKMAN A., GOWANS J. L. THE PRODUCTION OF MACROPHAGES IN THE RAT. Br J Exp Pathol. 1965 Feb;46:50–61. [PMC free article] [PubMed] [Google Scholar]
- WAKSMAN B. H., MATOLTSY M. The effect of tuberculin on peritoneal exudate cells of sensitized guinea pigs in surviving cell culture. J Immunol. 1958 Sep;81(3):220–234. [PubMed] [Google Scholar]
- Ward P. A., Remold H. G., David J. R. Leukotactic factor produced by sensitized lymphocytes. Science. 1969 Mar 7;163(3871):1079–1081. doi: 10.1126/science.163.3871.1079. [DOI] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Allergenicity of mycobacterial ribosomal and ribonucleic acid preparations in mice and guinea pigs. J Bacteriol. 1969 Jan;97(1):134–139. doi: 10.1128/jb.97.1.134-139.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]