Abstract
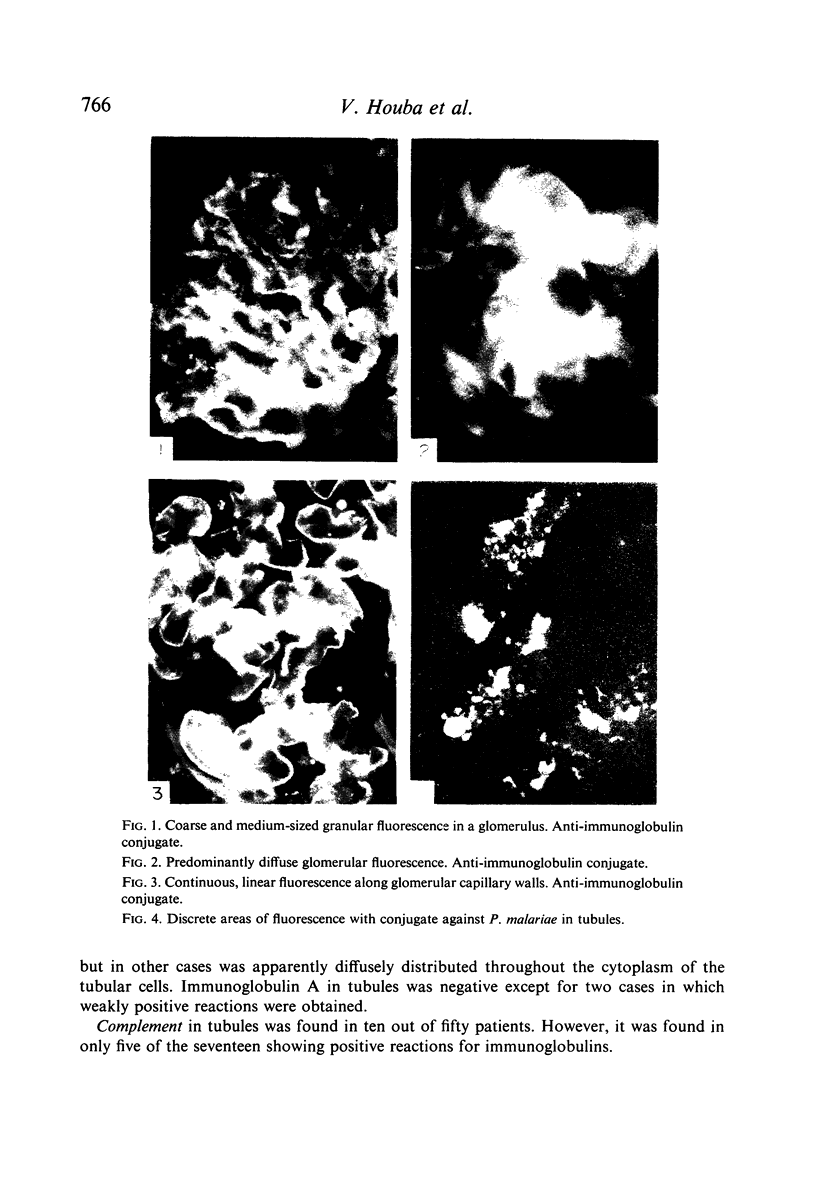
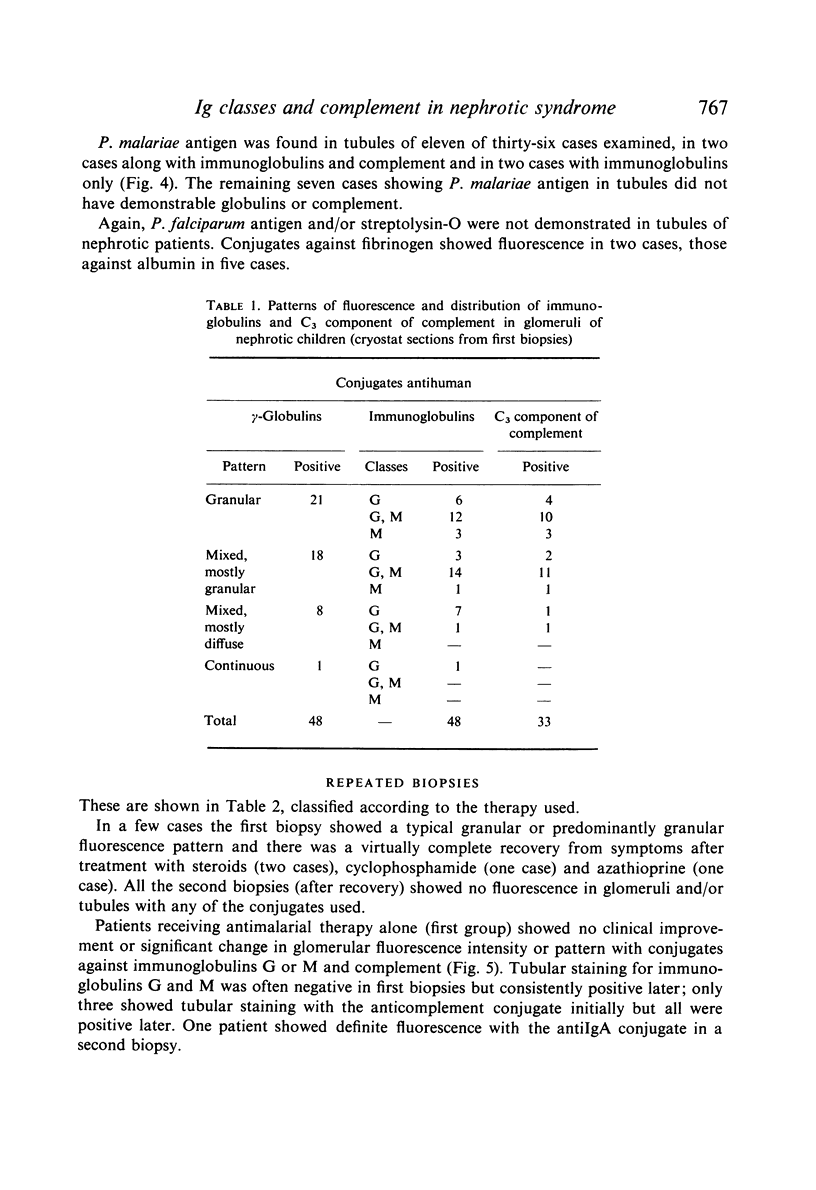
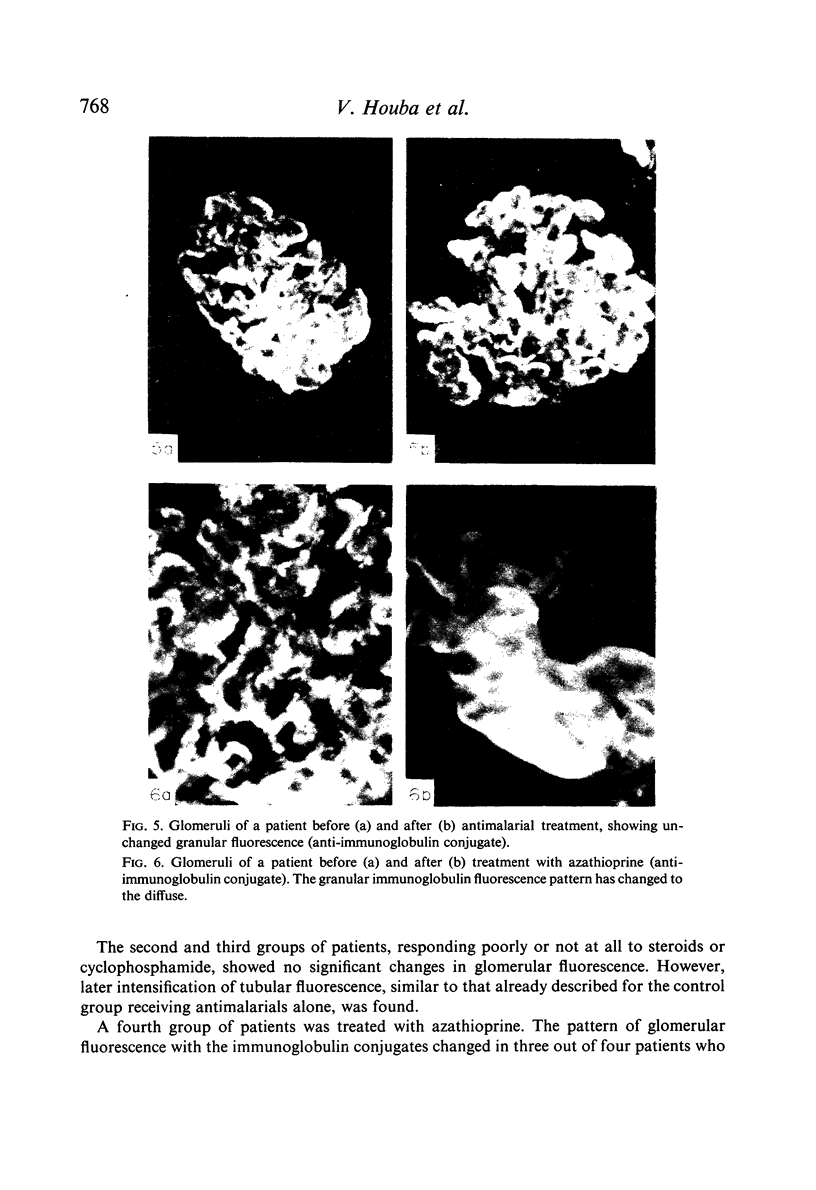
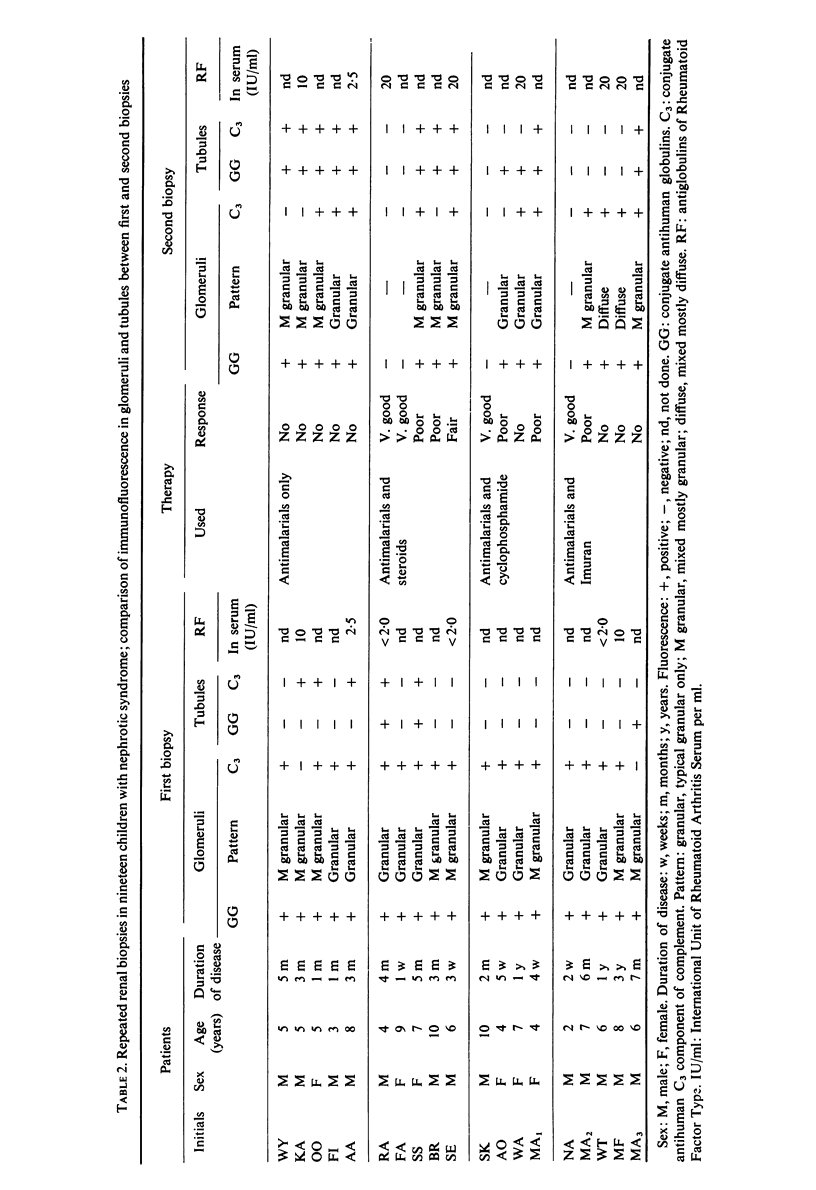
Pretreatment diagnostic renal biopsies from fifty Nigerian children with the nephrotic syndrome were investigated by immunofluorescence for immunoglobulin classes, complement and specific antigens. Nineteen of these were re-examined after an interval of 10–15 months. Forty-eight first biopsies were positive for bound γ-globulins, usually IgM and IgG but sometimes for one of these alone; IgA was not detected. Thirty-three were positive for bound complement (C3 component). IgM was associated with granular deposits and complement, IgG with both granular and continuous deposits, the latter usually lacking complement. Plasmodium malariae antigen was found in nine of thirty-six cases examined; no P. falciparum or streptolysin-O antigens were observed. Immunoglobulins G and M were found in tubules in seventeen of the fifty patients, in five together with complement. P. malariae antigen was observed in tubules in eleven of thirty-six cases.
Repeat biopsies from four patients who had recovered were negative with all reagents. Patients on anti-malarial therapy only, and those responding poorly to steroids or cyclophosphamide, showed no significant change in glomerular fluorescence, but a higher incidence of tubular fluorescence was noted in second biopsies. In patients with a poor response to Imuran treatment the pattern of glomerular fluorescence changed from granular to diffuse and tubular staining was not observed. In some patients increased levels of antiglobulins (rheumatoid factor type) were detected in later sera. The nature of the bound immunoglobulins was confirmed by elution of complexes and immunodiffusion.
It is suggested that an antigen–antibody complex with P. malariae antigen can produce renal damage with liberation of autoantigens which have the capacity to initiate self-perpetuating autoimmune disease.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeniyi A., Hendrickse R. G., Houba V. Selectivity of proteinuria and response to prednisolone or immunosuppressive drugs in children with malarial nephrosis. Lancet. 1970 Mar 28;1(7648):644–648. doi: 10.1016/s0140-6736(70)90885-8. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Hendrickse R. G., Edington G. M., Houba V., De Petris S., Adeniyi A. Immune complexes in the nephrotic syndrome of African children. Lancet. 1969 Jun 21;1(7608):1232–1238. doi: 10.1016/s0140-6736(69)92116-3. [DOI] [PubMed] [Google Scholar]
- Anderson S. G., Bentzon M. W., Houba V., Krag P. International reference preparation of rheumatoid arthritis serum. Bull World Health Organ. 1970;42(2):311–318. [PMC free article] [PubMed] [Google Scholar]
- Barabas A. Z., Elson C. J., Weir D. M. The serology of autologous immune complex nephritis in the rat. Clin Exp Immunol. 1969 Mar;4(3):345–351. [PMC free article] [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. I. Identification and isolation of the pathogenetic antigen. J Exp Med. 1968 Mar 1;127(3):555–572. doi: 10.1084/jem.127.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington T. S., Lee S., Dixon F. J. Persistence of the autoimmune pathogenetic process in experimental autologous immune complex nephritis. J Immunol. 1969 Sep;103(3):528–536. [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- GIGLIOLI G. Malaria and renal disease, with special reference to British Guiana. I. Introduction. Ann Trop Med Parasitol. 1962 Apr;56:101–109. doi: 10.1080/00034983.1962.11686096. [DOI] [PubMed] [Google Scholar]
- GIGLIOLI G. Malaria and renal disease, with special reference to British Guiana. II. The effect of malaria eradication on the incidence of renal disease in British Guiana. Ann Trop Med Parasitol. 1962 Jul;56:225–241. doi: 10.1080/00034983.1962.11686115. [DOI] [PubMed] [Google Scholar]
- Glassock R. J., Edgington T. S., Watson J. I., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. II. The pathogenetic mechanism. J Exp Med. 1968 Mar 1;127(3):573–588. doi: 10.1084/jem.127.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg L. S., Barnett E. V. The role of rheumatoid (antiglobulin) factors in hemolytic anemia). Ann N Y Acad Sci. 1969 Dec 10;168(1):122–125. doi: 10.1111/j.1749-6632.1969.tb43101.x. [DOI] [PubMed] [Google Scholar]
- HEYMANN W., HACKEL D. B., HARWOOD S., WILSON S. G., HUNTER J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959 Apr;100(4):660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- Henney C. S. Structural and conformational specificity of the antigen for rheumatoid factor. Ann N Y Acad Sci. 1969 Dec 10;168(1):52–62. doi: 10.1111/j.1749-6632.1969.tb43094.x. [DOI] [PubMed] [Google Scholar]
- Heymann W., Kmetec E. P., Wilson S. G., Hunter J. L., Hackel D. B., Okuda R., Cuppage F. Experimental autoimmune renal disease in rats. Ann N Y Acad Sci. 1965 Jun 30;124(1):310–322. doi: 10.1111/j.1749-6632.1965.tb18966.x. [DOI] [PubMed] [Google Scholar]
- Houba V., Allison A. C. M-antiglobulins (rheumatoid-factor-like globulins) and other gamma-globulins in relation to tropical parasitic infections. Lancet. 1966 Apr 16;1(7442):848–852. doi: 10.1016/s0140-6736(66)90186-3. [DOI] [PubMed] [Google Scholar]
- KARK R. M., MUEHRCKE R. C. Biopsy of kidney in prone position. Lancet. 1954 May 22;266(6821):1047–1049. doi: 10.1016/s0140-6736(54)91618-9. [DOI] [PubMed] [Google Scholar]
- Kibukamusoke J. W., Hutt M. S. Histological features of the nephrotic syndrome associated with quartan malaria. J Clin Pathol. 1967 Mar;20(2):117–123. doi: 10.1136/jcp.20.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A. F., Jr, Drummond K. N., Good R. A., Vernier R. L. Acute poststreptococcal glomerulonephritis: immune deposit disease. J Clin Invest. 1966 Feb;45(2):237–248. doi: 10.1172/JCI105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. T., Kano K., Milgrom F. In vivo interactions of antiglobulin factors. Ann N Y Acad Sci. 1969 Dec 10;168(1):140–145. doi: 10.1111/j.1749-6632.1969.tb43103.x. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Kibukamusoke J. W. Evidence for soluble immune complexes in the pathogenesis of the glomerulonephritis of quartan malaria. Lancet. 1969 Feb 8;1(7589):283–285. doi: 10.1016/s0140-6736(69)91038-1. [DOI] [PubMed] [Google Scholar]
- Weigle W. O., Nakamura R. M. Perpetuation of autoimmune thyroiditis and production of secondary renal lesions following periodic injections of aqueous preparations of altered thyroglobulin. Clin Exp Immunol. 1969 Jun;4(6):645–657. [PMC free article] [PubMed] [Google Scholar]


