Abstract
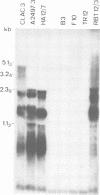
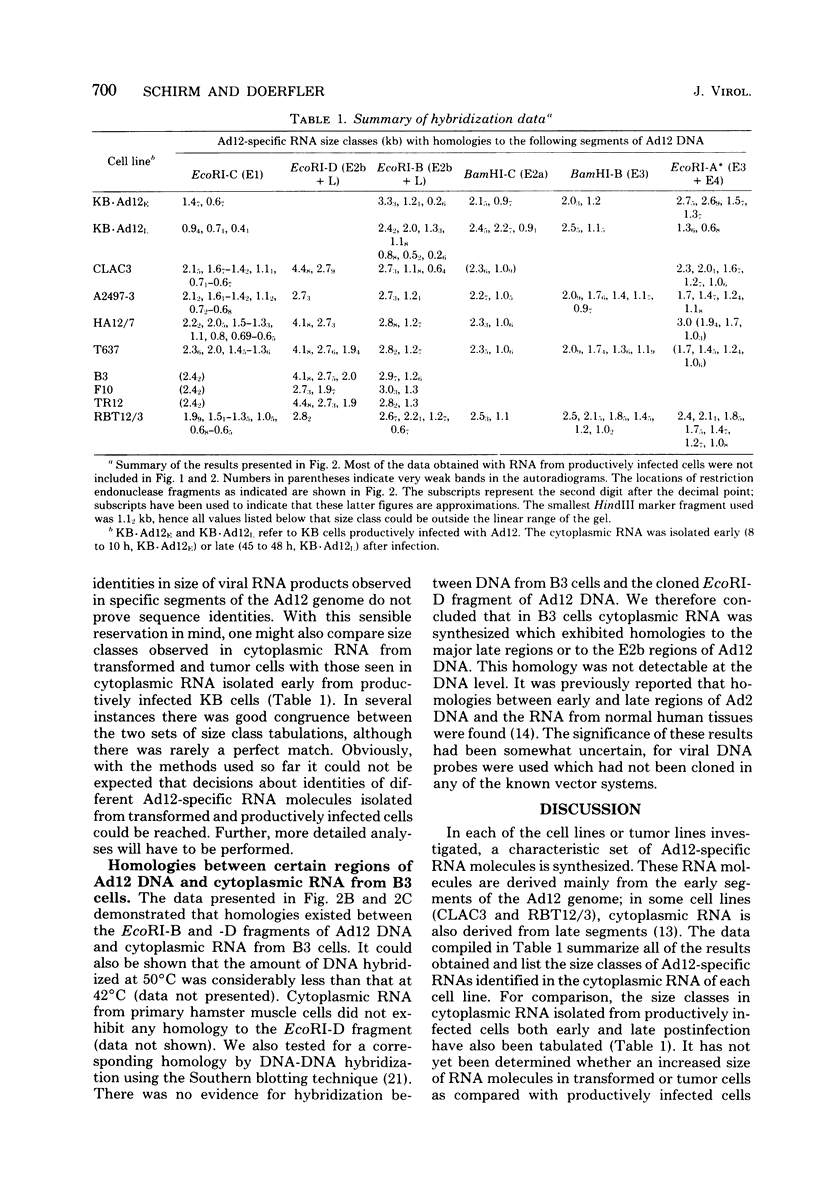
The expression as cytoplasmic RNA of integrated human adenovirus type 12 (Ad12) DNA in transformed and tumor cell lines and in revertants was investigated. The transformed and tumor cells contained multiple copies of the viral genome, 3 to 22 copies per cell in different cell lines. The integrated Ad12 DNA molecules persisted intact or nearly intact and in most cases colinear with the virion DNA. In the revertant cell lines, which were derived from cell line T637 (22 copies of Ad12 DNA per cell), all of the Ad12 DNA molecules were lost (line F10) or only one copy and a fraction of a second copy persisted (line TR12). The size classes and map locations of Ad12-specific cytoplasmic RNAs in three Ad12-transformed hamster cell lines (T637, HA12/7, and A2497-3), in two revertant lines (F10 and TR12), in one Ad12-induced hamster (CLAC3), and in one rat brain tumor line (RBT12/3) were determined. Cytoplasmic RNA from uninfected B3 hamster cells and from human KB cells productively infected with Ad12 served as controls. In the latter control experiments, the RNA was isolated early or late postinfection. With respect to the amounts of Ad12-specific RNAs detected in cytoplasmic RNA from various Ad12-transformed or Ad12-induced tumor cell lines, we could not establish any correlations to the number of Ad12 genome copies integrated into the cellular DNAs. Thus, the expression of the integrated viral genomes in these lines was regulated by mechanisms more complicated than simple gene dosage effects. Using cloned fragments of Ad12 DNA as hybridization probes, we analyzed the cytoplasmic RNAs from the cell lines mentioned by electrophoresis on agarose gels, blotting, and DNA-RNA hybridization. For each transformed and tumor cell line, except for the revertants, several size classes of Ad12-specific cytoplasmic RNA were detected for the early E1, E2, and E4 regions of Ad12 DNA. Some of these size classes were similar but not identical to those observed in cytoplasmic RNA isolated early from human KB cells productively infected with Ad12. Only cell lines A2497-3, T637, and RBT12/3 contained several size classes of cytoplasmic RNA homologous to the E3 region of Ad12 DNA. Weak homologies to the E1 region of Ad12 DNA were also detected in the revertant lines F10 and TR12. Late regions of Ad12 DNA were expressed as cytoplasmic RNA in cell lines CLAC3 and RBT12/3. Weak homologies were detected between certain segments of the Ad12 genome (the EcoRI-B, -C, and -D fragments) and the cytoplasmic RNA from uninfected hamster cells. These homologies had no apparent counterpart at the level of DNA, perhaps because these homologies could be detected only due to an overrepresentation of RNA sequences. In preliminary experiments, we failed to detect the expression as cytoplasmic RNA of the so-called virus-associated RNA in transformed and tumor cell lines. Virus-associated RNA represents a population of low-molecular-weight RNAs that map at around 30 fractional length units on the viral genome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Mulder C., Fleckenstein B. Methylation of Herpesvirus saimiri DNA in lymphoid tumor cell lines. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3839–3843. doi: 10.1073/pnas.76.8.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Stabel S., Ibelgaufts H., Sutter D., Neumann R., Groneberg J., Scheidtmann K. H., Deuring R., Winterhoff U. Selectivity in integration sites of adenoviral DNA. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):551–564. doi: 10.1101/sqb.1980.044.01.057. [DOI] [PubMed] [Google Scholar]
- Eick D., Stabel S., Doerfler W. Revertants of adenovirus type 12-transformed hamster cell line T637 as tools in the analysis of integration patterns. J Virol. 1980 Oct;36(1):41–49. doi: 10.1128/jvi.36.1.41-49.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg J., Doerfler W. Revertants of adenovirus type-12-transformed hamster cells have lost part of the viral genomes. Int J Cancer. 1979 Jul 15;24(1):67–74. doi: 10.1002/ijc.2910240112. [DOI] [PubMed] [Google Scholar]
- Groneberg J., Sutter D., Soboll H., Doerfler W. Morphological revertants of adenovirus type 12-transformed hamster cells. J Gen Virol. 1978 Sep;40(3):635–645. doi: 10.1099/0022-1317-40-3-635. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Rao P. Y., Mitsialis S. A., Katz R. Modification of avian sarcoma proviral DNA sequences in nonpermissive XC cells but not in permissive chicken cells. J Virol. 1980 May;34(2):569–572. doi: 10.1128/jvi.34.2.569-572.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibelgaufts H., Doerfler W., Scheidtmann K. H., Wechsler W. Adenovirus type 12-induced rat tumor cells of neuroepithelial origin: persistence and expression of the viral genome. J Virol. 1980 Jan;33(1):423–437. doi: 10.1128/jvi.33.1.423-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. W., Kinross J., Maitland N., Norval M. Normal human tissues contain RNA and antigens related to infectious adenovirus type 2. Nature. 1979 Jan 25;277(5694):274–279. doi: 10.1038/277274a0. [DOI] [PubMed] [Google Scholar]
- Neumann R., Doerfler W. Integration of adenovirus type 2 DNA at a limited number of cellular sites in productively infected cells. J Virol. 1981 Mar;37(3):887–892. doi: 10.1128/jvi.37.3.887-892.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin J., Scheidtmann K. H., Greenberg R., Westphal M., Doerfler W. Transcription of the genome of adenovirus type 12. III. Maps of stable RNA from productively infected human cells and abortively infected and transformed hamster cells. J Virol. 1976 Nov;20(2):355–372. doi: 10.1128/jvi.20.2.355-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Fujinaga K. Mapping of adenovirus 12 mRNA's transcribed from the transforming region. J Virol. 1980 Dec;36(3):639–651. doi: 10.1128/jvi.36.3.639-651.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Ortin J., Doerfler W. Transcription of the genome of adenovirus type 12. Viral mRNA in productively infected KB cells. Eur J Biochem. 1975 Oct 15;58(2):283–290. doi: 10.1111/j.1432-1033.1975.tb02374.x. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stabel S., Doerfler W., Friis R. R. Integration sites of adenovirus type 12 DNA in transformed hamster cells and hamster tumor cells. J Virol. 1980 Oct;36(1):22–40. doi: 10.1128/jvi.36.1.22-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl W. A., Rouse H., Teets K., Schlesinger R. W. The response of BHK21 cells to infection with type 12 adenovirus. 3. Transformation and restricted replication of superinfecting type 2 adenovirus. Arch Gesamte Virusforsch. 1970;31(1):93–112. doi: 10.1007/BF01241669. [DOI] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated viral DNA sequences in hamster cells transformed by adenovirus 12. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):565–568. doi: 10.1101/sqb.1980.044.01.058. [DOI] [PubMed] [Google Scholar]
- Sutter D., Westphal M., Doerfler W. Patterns of integration of viral DNA sequences in the genomes of adenovirus type 12-transformed hamster cells. Cell. 1978 Jul;14(3):569–585. doi: 10.1016/0092-8674(78)90243-x. [DOI] [PubMed] [Google Scholar]
- Tiemer D., Enquist L., Leder P. Improved derivative of a phage lambda EK2 vector for cloning recombinant DNA. Nature. 1976 Oct 7;263(5577):526–527. doi: 10.1038/263526a0. [DOI] [PubMed] [Google Scholar]
- Tjia S. T., Carstens E. B., Doerfler W. Infection of Spodoptera frugiperda cells with Autographa californica nuclear polyhedrosis virus II. The viral DNA and the kinetics of its replication. Virology. 1979 Dec;99(2):399–409. doi: 10.1016/0042-6822(79)90018-7. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Neumann R., Kuhlmann I., Sutter D., Doerfler W. DNA methylation and viral gene expression in adenovirus-transformed and -infected cells. Nucleic Acids Res. 1980 Jun 11;8(11):2461–2473. doi: 10.1093/nar/8.11.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Fujinaga K. Unique species of mRNA from adenovirus type 7 early region 1 in cells transformed by adenovirus type 7 DNA fragment. J Virol. 1980 Nov;36(2):337–352. doi: 10.1128/jvi.36.2.337-352.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Interactions of adenovirus type 12 with host cell chromosomes. Prog Exp Tumor Res. 1973;18:240–259. doi: 10.1159/000393169. [DOI] [PubMed] [Google Scholar]