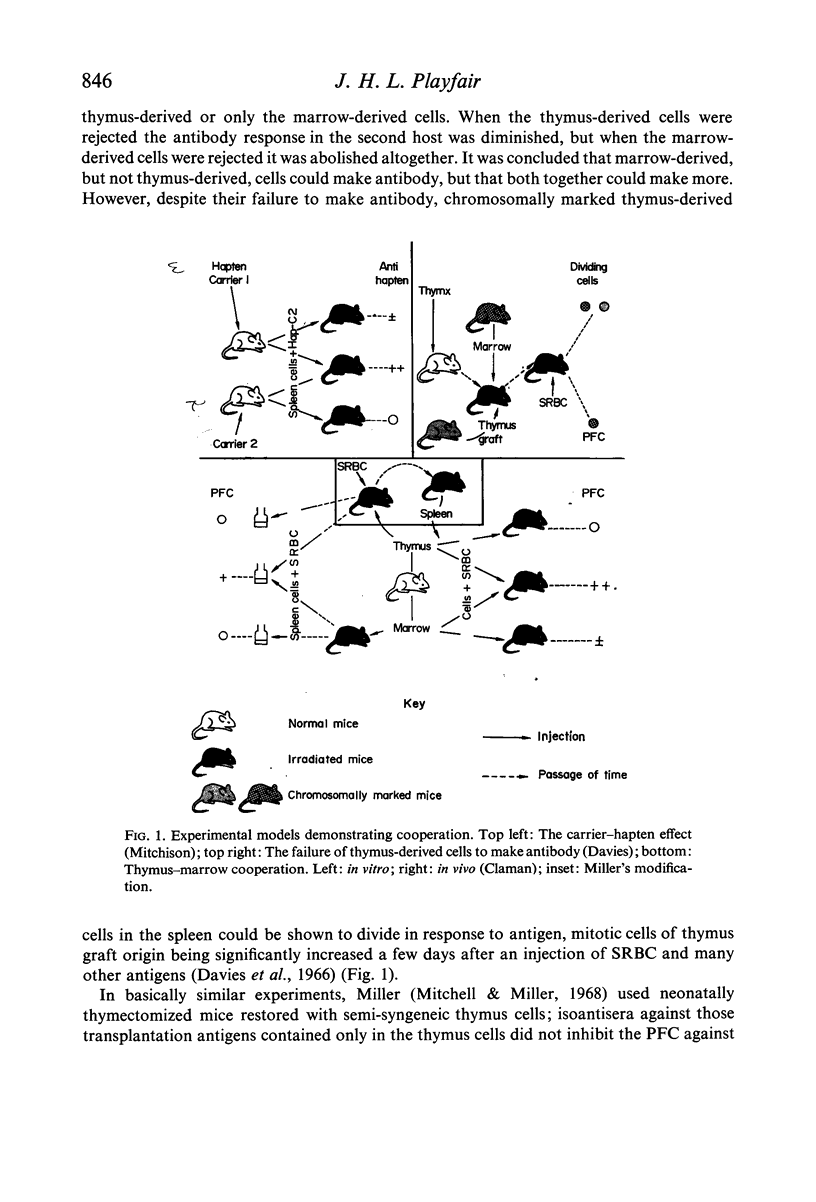
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Rose B., Richter M. Cells involved in the immune response. 8. The relationship between the loss and reappearance of antigen-reactive cells and immune responsiveness after irradiation of normal adult rabbits. J Exp Med. 1969 Oct 1;130(4):867–876. doi: 10.1084/jem.130.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong W. D., Diener E., Shellam G. R. Antigen-reactive cells in normal, immunized, and tolerant mice. J Exp Med. 1969 Feb 1;129(2):393–410. doi: 10.1084/jem.129.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER A. J., McCULLOCH E. A., TILL J. E. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963 Feb 2;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B., SPARROW E. M. Quantitative studies on tissue transplantation immunity. I. The survival times of skin homografts exchanged between members of different inbred strains of mice. Proc R Soc Lond B Biol Sci. 1954 Dec 15;143(910):43–58. doi: 10.1098/rspb.1954.0053. [DOI] [PubMed] [Google Scholar]
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Baker P. J., Landy M. Brevity of the inductive phase in the immune response of mice to capsular polysaccharide antigens. J Immunol. 1967 Oct;99(4):687–694. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. II. Studies on the relative rate of antibody synthesis and release by antibody-producing cells. Immunology. 1971 Apr;20(4):481–492. [PMC free article] [PubMed] [Google Scholar]
- Brody T. Identification of two cell populations required for mouse immunocompetence. J Immunol. 1970 Jul;105(1):126–138. [PubMed] [Google Scholar]
- Bussard A. E., Lurie M. Primary antibody response in vitro in peritoneal cells. J Exp Med. 1967 May 1;125(5):873–892. doi: 10.1084/jem.125.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUDKOWICZ G., BENNETT M., SHEARER G. M. PLURIPOTENT STEM CELL FUNCTION OF THE MOUSE MARROW "LYMPHOCYTE". Science. 1964 May 15;144(3620):866–868. doi: 10.1126/science.144.3620.866. [DOI] [PubMed] [Google Scholar]
- Cantor H., Asofsky R. Synergy among lymphoid cells mediating the graft-versus-host response. II. Synergy in graft-versus-host reactions produced by Balb-c lymphoid cells of differing anatomic origin. J Exp Med. 1970 Feb;131(2):235–246. doi: 10.1084/jem.131.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Cellular sites of immunologic unresponsiveness. Proc Natl Acad Sci U S A. 1970 Mar;65(3):551–556. doi: 10.1073/pnas.65.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A. Immunologic complementation between thymus and marrow cells--a model for the two-cell theory of immunocompetence. Transplant Rev. 1969;1:92–113. doi: 10.1111/j.1600-065x.1969.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A., Triplett R. F. Thymus-marrow cell combinations. Synergism in antibody production. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1167–1171. doi: 10.3181/00379727-122-31353. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Perey D. Y., McKneally M. F., Gabrielsen A. E., Sutherland D. E., Good R. A. A mammalian equivalent of the avian bursa of Fabricius. Lancet. 1966 Jun 25;1(7452):1388–1391. doi: 10.1016/s0140-6736(66)90300-x. [DOI] [PubMed] [Google Scholar]
- Davies A. J., Leuchars E., Wallis V., Koller P. C. The mitotic response of thymus-derived cells to antigenic stimulus. Transplantation. 1966 Jul;4(4):438–451. doi: 10.1097/00007890-196607000-00008. [DOI] [PubMed] [Google Scholar]
- Doenhoff M. J., Davies A. J., Leuchars E., Wallis V. The thymus and circulating lymphocytes of mice. Proc R Soc Lond B Biol Sci. 1970 Oct 13;176(1042):69–85. doi: 10.1098/rspb.1970.0035. [DOI] [PubMed] [Google Scholar]
- Duffus W. P., Allan D. A study of the ontogeny of specific immune responsiveness amongst circulating leucocytes in the chicken. Immunology. 1969 Mar;16(3):337–347. [PMC free article] [PubMed] [Google Scholar]
- Dumonde D. C., Wolstencroft R. A., Panayi G. S., Matthew M., Morley J., Howson W. T. "Lymphokines": non-antibody mediators of cellular immunity generated by lymphocyte activation. Nature. 1969 Oct 4;224(5214):38–42. doi: 10.1038/224038a0. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Cooperation of immune lymphoid cells with macrophages in tumour immunity. Nature. 1970 Nov 14;228(5272):620–622. doi: 10.1038/228620a0. [DOI] [PubMed] [Google Scholar]
- FORD C. E., HAMERTON J. L., BARNES D. W., LOUTIT J. F. Cytological identification of radiation-chimaeras. Nature. 1956 Mar 10;177(4506):452–454. doi: 10.1038/177452a0. [DOI] [PubMed] [Google Scholar]
- FORD C. E., MICKLEM H. S. The thymus and lymph-nodes in radiation chimaeras. Lancet. 1963 Feb 16;1(7277):359–362. doi: 10.1016/s0140-6736(63)91385-0. [DOI] [PubMed] [Google Scholar]
- GOOD R. A., KELLY W. D., ROTSTEIN J., VARCO R. L. Immunological deficiency diseases. Agammaglobulinemia, hypogammaglobulinemia, Hodgkin's disease and sarcoidosis. Prog Allergy. 1962;6:187–319. [PubMed] [Google Scholar]
- GOWANS J. L. The fate of parental strain small lymphocytes in F1 hybrid rats. Ann N Y Acad Sci. 1962 Oct 24;99:432–455. doi: 10.1111/j.1749-6632.1962.tb45326.x. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol. 1959 Apr 23;146(1):54–69. doi: 10.1113/jphysiol.1959.sp006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Wallis V., Davies A. J., Leuchars E. Inactivation of thymus cells after multiple injections of antigen. Nature. 1968 Apr 27;218(5139):380–381. doi: 10.1038/218380a0. [DOI] [PubMed] [Google Scholar]
- Gilmour D. G., Theis G. A., Thorbecke G. J. Transfer of antibody production with cells from bursa of Fabricius. J Exp Med. 1970 Jul 1;132(1):134–147. doi: 10.1084/jem.132.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Asanuma Y., Battisto J. R., Hardy M. A., Quint J., White A. Influence of thymosin on cell-mediated and humoral immune responses in normal and in immunologically deficient mice. J Immunol. 1970 Feb;104(2):359–366. [PubMed] [Google Scholar]
- Greaves M. F., Torrigiani G., Roitt I. M. Blocking of the lymphocyte receptor site for cell mediated hypersensitivity and transplantation reactions by anti-light chain sera. Nature. 1969 May 31;222(5196):885–886. doi: 10.1038/222885a0. [DOI] [PubMed] [Google Scholar]
- Gregory C. J., Lajtha L. G. Kinetic study of the production of antibody-forming cells from their precursors. Nature. 1968 Jun 15;218(5146):1079–1081. doi: 10.1038/2181079a0. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H., PARROTT D. M., EAST J. STUDIES ON GLOBULIN AND ANTIBODY PRODUCTION IN MICE THYMECTOMIZED AT BIRTH. Immunology. 1964 Jul;7:419–439. [PMC free article] [PubMed] [Google Scholar]
- Hanaoka M., Nomoto K., Waksman B. H. Appendix and gamma M-antibody formation. I. Immune response and tolerance to bovine gamma globulin in irradiated, appendix-shielded rabbits. J Immunol. 1970 Mar;104(3):616–625. [PubMed] [Google Scholar]
- Hartmann K. U. Induction of a hemolysin response in vitro. Interaction of cells of bone marrow origin and thymic origin. J Exp Med. 1970 Dec 1;132(6):1267–1278. doi: 10.1084/jem.132.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C., Jerne N. K. Competition of 19S and 7S antigen receptors in the regulation of the primary immune response. J Exp Med. 1968 Jul 1;128(1):133–152. doi: 10.1084/jem.128.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh E. M., Harris J. E. Macrophage-lymphocyte interaction in the antigen-induced blastogenic response of human peripheral blood leukocytes. J Immunol. 1968 Jun;100(6):1184–1194. [PubMed] [Google Scholar]
- Isaković K., Smith S. B., Waksman B. H. Role of the thymus in tolerance. I. Tolerance to bovine gamma globulin in thymectomized, irradiated rats grafted with thymus from tolerant donors. J Exp Med. 1965 Dec 1;122(6):1103–1123. doi: 10.1084/jem.122.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Jacobson E. B., L'age-Stehr J., Herzenberg L. A. Immunological memory in mice. II. Cell interactions in the secondary immune response studies by means of immunoglobulin allotype markers. J Exp Med. 1970 Jun 1;131(6):1109–1120. doi: 10.1084/jem.131.6.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. C., Treadwell P. E., Lennox E. S. Antigen-specific synergism in the immune response of irradiated mice given marrow cells and peritoneal cavity cells or extracts. J Exp Med. 1970 Aug 1;132(2):353–367. doi: 10.1084/jem.132.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., Herzenberg L. A. Congenic mouse strains with different immunoglobulin allotypes. I. Breeding scheme, histocompatibility tests, and kinetics of gamma G2a-globulin production by transferred cells for C3H.SW and its congenic partner CWB/5. Transplantation. 1967 Nov;5(6):1484–1495. doi: 10.1097/00007890-196711000-00013. [DOI] [PubMed] [Google Scholar]
- Lindenmann J., Klein P. A. Viral oncolysis: increased immunogenicity of host cell antigen associated with influenza virus. J Exp Med. 1967 Jul 1;126(1):93–108. doi: 10.1084/jem.126.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischner H. W., DiGeorge A. M. Role of the thymus in humoral immunity. Lancet. 1969 Nov 15;2(7629):1044–1049. doi: 10.1016/s0140-6736(69)90647-3. [DOI] [PubMed] [Google Scholar]
- Lubaroff D. M., Waksman B. H. Bone marrow as a source of cells in reactions of cellular hypersensitivity. I. Passive transfer of tuberculin sensitivity in syngeneic systems. J Exp Med. 1968 Dec 1;128(6):1425–1435. doi: 10.1084/jem.128.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. F. Immunological function of the thymus. Lancet. 1961 Sep 30;2(7205):748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- Marbrook J. Primary immune response in cultures of spleen cells. Lancet. 1967 Dec 16;2(7529):1279–1281. doi: 10.1016/s0140-6736(67)90393-5. [DOI] [PubMed] [Google Scholar]
- Mason S., Warner N. L. The immunoglobulin nature of the antigen recognition site on cells mediating transplantation immunity and delayed hypersentivity. J Immunol. 1970 Mar;104(3):762–765. [PubMed] [Google Scholar]
- McConnell I., Munro A., Gurner B. W., Coombs R. R. Studies on actively allergized cells. I. The cyto-dynamics and morphology of rosete-forming lymph node cells in mice and inhibition of rosette-formation with antibody to mouse immunoglobulins. Int Arch Allergy Appl Immunol. 1969;35(3):209–227. [PubMed] [Google Scholar]
- McDevitt H. O., Tyan M. L. Genetic control of the antibody response in inbred mice. Transfer of response by spleen cells and linkage to the major histocompatibility (H-2) locus. J Exp Med. 1968 Jul 1;128(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., McCullagh P. J., Gowans J. L. The role of lymphocytes in antibody formation. I. Restoration of the haemolysin response in x-irradiated rats with lymphocytes from normal and immunologically tolerant donors. Proc R Soc Lond B Biol Sci. 1967 Sep 12;168(1012):229–243. doi: 10.1098/rspb.1967.0063. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):801–820. doi: 10.1084/jem.128.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Cell to cell interaction in the immune response. V. Target cells for tolerance induction. J Exp Med. 1970 Apr 1;131(4):675–699. doi: 10.1084/jem.131.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):821–837. doi: 10.1084/jem.128.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E. A requirement for two cell types for antibody formation in vitro. Science. 1967 Dec 22;158(3808):1573–1575. doi: 10.1126/science.158.3808.1573. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Fitch F. W., Rowley D. A., Davies A. J. Cellular deficit in thymectomized mice. Nature. 1970 Jan 17;225(5229):276–277. doi: 10.1038/225276a0. [DOI] [PubMed] [Google Scholar]
- NOTA N. R., LIACOPOULOS-BRIOT M., STIFFEL C., BIOZZI G. L'IMMUNO-CYTO-ADH'ERENCE: UNE M'ETHODE SIMPLE ET QUANTITATIVE POUR L'ETUDE IN VITRO DES CELLULES PRODUCTRICES D'ANTICORPS. C R Hebd Seances Acad Sci. 1964 Aug 3;259:1277–1280. [PubMed] [Google Scholar]
- Nossal G. J., Cunningham A., Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. 3. Chromosomal marker analysis of single antibody-forming cells in reconstituted, irradiated, or thymectomized mice. J Exp Med. 1968 Oct 1;128(4):839–853. doi: 10.1084/jem.128.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., Papermaster B. W., Cole L. J. Focal antibody production by transferred spleen cells in irradiated mice. Science. 1965 Aug 27;149(3687):998–1000. doi: 10.1126/science.149.3687.998. [DOI] [PubMed] [Google Scholar]
- Playfair J. H. Specific tolerance to sheep erythrocytes in mouse bone marrow cells. Nature. 1969 May 31;222(5196):882–883. doi: 10.1038/222882a0. [DOI] [PubMed] [Google Scholar]
- Potworowski E. F., Nairn R. C. Origin and fate of a thymocyte-specific antigen. Immunology. 1967 Dec;13(6):597–602. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Nase S., Mitchison N. A. Mouse specific bone marrow-derived lymphocyte antigen as a marker for thymus-independent lymphocytes. Nature. 1971 Mar 5;230(5288):50–51. doi: 10.1038/230050a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Role of thymus-derived lymphocytes in the secondary humoral immune response in mice. Nature. 1970 Jun 27;226(5252):1257–1258. doi: 10.1038/2261257a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Wortis H. H. Thymus dependence of theta-bearing cells in the peripheral lymphoid tissues of mice. Immunology. 1970 Jun;18(6):931–942. [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K., Rottländer E., Peltre G., Müller B. The immune response to a hybrid protein molecule; specificity of secondary stimulation and of tolerance induction. J Exp Med. 1967 Oct 1;126(4):581–606. doi: 10.1084/jem.126.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K., Schirrmacher V., Nase S., Jerne N. K. The requirement of more than one antigenic determinant for immunogenicity. J Exp Med. 1969 Jun 1;129(6):1131–1143. doi: 10.1084/jem.129.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M., Abdou N. I. Cells involved in the immune response. VII. The demonstration, using allotypic markers, of antibody formation by irradiation-resistant cells of irradiated rabbits injected with normal allogeneic bone marrow cells and sheep erythrocytes. J Exp Med. 1969 Jun 1;129(6):1261–1273. doi: 10.1084/jem.129.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M. Cells involved in cell-mediated and transplantation immunity. II. A consideration of the functional identity of the cells involved in both humoral and cell-mediated immunity: a phylogenetic approach. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1127–1135. doi: 10.1073/pnas.66.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitt I. M., Greaves M. F., Torrigiani G., Brostoff J., Playfair J. H. The cellular basis of immunological responses. A synthesis of some current views. Lancet. 1969 Aug 16;2(7616):367–371. doi: 10.1016/s0140-6736(69)92712-3. [DOI] [PubMed] [Google Scholar]
- SELL S., GELL P. G. STUDIES ON RABBIT LYMPHOCYTES IN VITRO. I. STIMULATION OF BLAST TRANSFORMATION WITH AN ANTIALLOTYPE SERUM. J Exp Med. 1965 Aug 1;122:423–440. doi: 10.1084/jem.122.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMONSEN M. Graft versus host reactions. Their natural history, and applicability as tools of research. Prog Allergy. 1962;6:349–467. [PubMed] [Google Scholar]
- Saunders G. C. Maturation of hemolysin-producing cell clones. I. The kinetics of the induction period of an in vitro hemolysin response to erythrocyte antigen. J Exp Med. 1969 Sep 1;130(3):543–556. doi: 10.1084/jem.130.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. Anti-theta antibodies for detecting thymus-dependent lymphocytes in the immune response of mice to SRBC. Nature. 1970 Jun 27;226(5252):1254–1256. doi: 10.1038/2261254a0. [DOI] [PubMed] [Google Scholar]
- Shortman K., Diener E., Russell P., Armstrong W. D. The role of nonlymphoid accessory cells in the immune response to different antigens. J Exp Med. 1970 Mar 1;131(3):461–482. doi: 10.1084/jem.131.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb U., Bauer W., Storb R., Fliedner T. M., Weiser R. S. Ultrastructure of rosette-forming cells in the mouse during the antibody response. J Immunol. 1969 Jun;102(6):1474–1485. [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Taylor R. B., Iverson G. M. Hapten competition and the nature of cell-cooperation in the antibody response. Proc R Soc Lond B Biol Sci. 1971 Jan 12;176(1045):393–418. doi: 10.1098/rspb.1971.0003. [DOI] [PubMed] [Google Scholar]
- Taylor R. B., Wortis H. H. Thymus dependence of antibody response: variation with dose of antigen and class of antibody. Nature. 1968 Nov 30;220(5170):927–928. doi: 10.1038/220927a0. [DOI] [PubMed] [Google Scholar]
- Trainin N., Small M., Globerson A. Immunocompetence of spleen cells from neonatally thymectomized mice conferred in vitro by a syngeneic thymus extract. J Exp Med. 1969 Oct 1;130(4):765–775. doi: 10.1084/jem.130.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentin J., Wolf N., Cheng V., Fahlberg W., Weiss D., Bonhag R. Antibody production by mice repopulated with limited numbers of clones of lymphoid cell precursors. J Immunol. 1967 Jun;98(6):1326–1337. [PubMed] [Google Scholar]
- WARNER N. L., SZENBERG A. THE IMMUNOLOGICAL FUNCTION OF THE BURSA OF FABRICIUS IN THE CHICKEN. Annu Rev Microbiol. 1964;18:253–268. doi: 10.1146/annurev.mi.18.100164.001345. [DOI] [PubMed] [Google Scholar]
- Watkins J. F., Chen L. Immunization of mice against Ehrlich ascites tumour using a hamster-Ehrlich ascites tumour hybrid cell line. Nature. 1969 Sep 6;223(5210):1018–1022. doi: 10.1038/2231018a0. [DOI] [PubMed] [Google Scholar]
- Weissman I. L. Thymus cell migration. J Exp Med. 1967 Aug 1;126(2):291–304. doi: 10.1084/jem.126.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigzell H., Mäkelä O. Separation of normal and immune lymphoid cells by antigen-coated coated columns. Antigen-binding characteristics of membrane antibodies as analyzed by hapten-protein antigens. J Exp Med. 1970 Jul 1;132(1):110–126. doi: 10.1084/jem.132.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. B., Munro A., Coombs R. R. Studies on actively allergized cells. II. Induction on guinea-pig lymphocytes of specific receptors to a soluble polypeptide antigen (rabbit IgG Fab'). Int Arch Allergy Appl Immunol. 1969;35(3):228–241. [PubMed] [Google Scholar]
- Wolf N. S., Trentin J. J. Hemopoietic colony studies. V. Effect of hemopoietic organ stroma on differentiation of pluripotent stem cells. J Exp Med. 1968 Jan 1;127(1):205–214. doi: 10.1084/jem.127.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P. L., Vazquez J., Czajkowski N. P., Rosenblatt M. A new method of active immunisation to autologous human tumour tissue. Lancet. 1967 Oct 28;2(7522):905–909. doi: 10.1016/s0140-6736(67)90229-2. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Till J. E., Siminovitch L., McCulloch E. A. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968 Mar 1;127(3):455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAALBERG O. B. A SIMPLE METHOD FOR DETECTING SINGLE ANTIBODY-FORMING CELLS. Nature. 1964 Jun 20;202:1231–1231. doi: 10.1038/2021231a0. [DOI] [PubMed] [Google Scholar]


