Abstract
The optimal conditions for assessing the rate of DNA synthesis by the incorporation of labelled thymidine in PHA-stimulated human lymphocytes were determined. Optimal labelling conditions could be sustained for only 3–4 hr. The exposure time was limited by both the failure to maintain saturating concentrations of exogenous thymidine and the deleterious affect of internal radiation. A linear incorporation of the label with time was not found to be a reliable criteria of optimal labelling conditions.
Full text
PDF


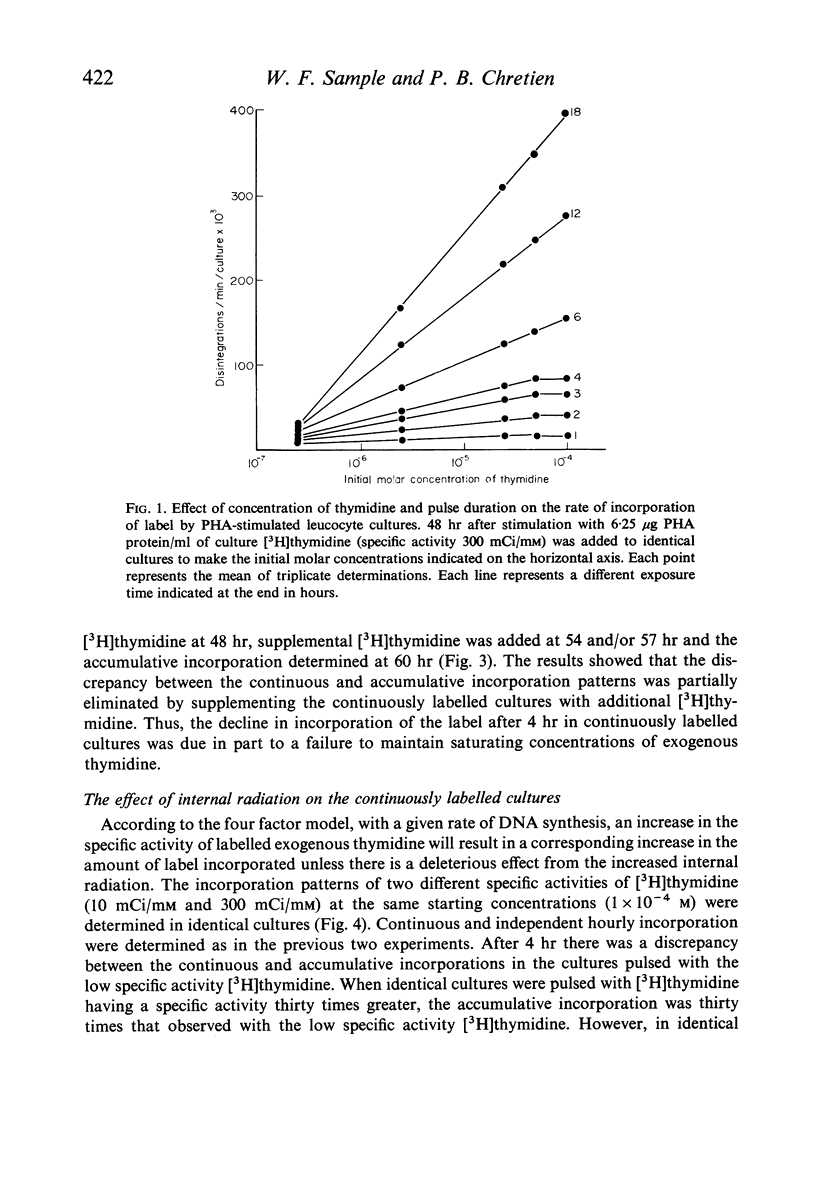
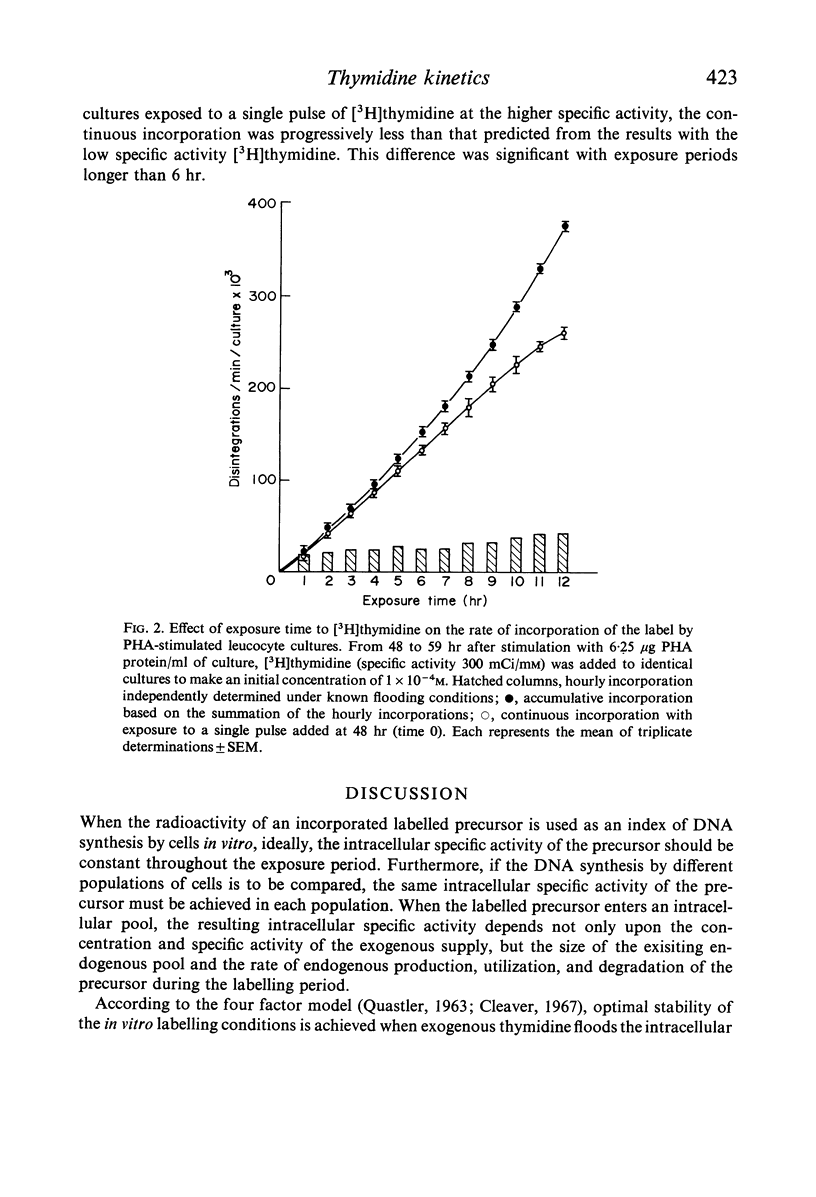
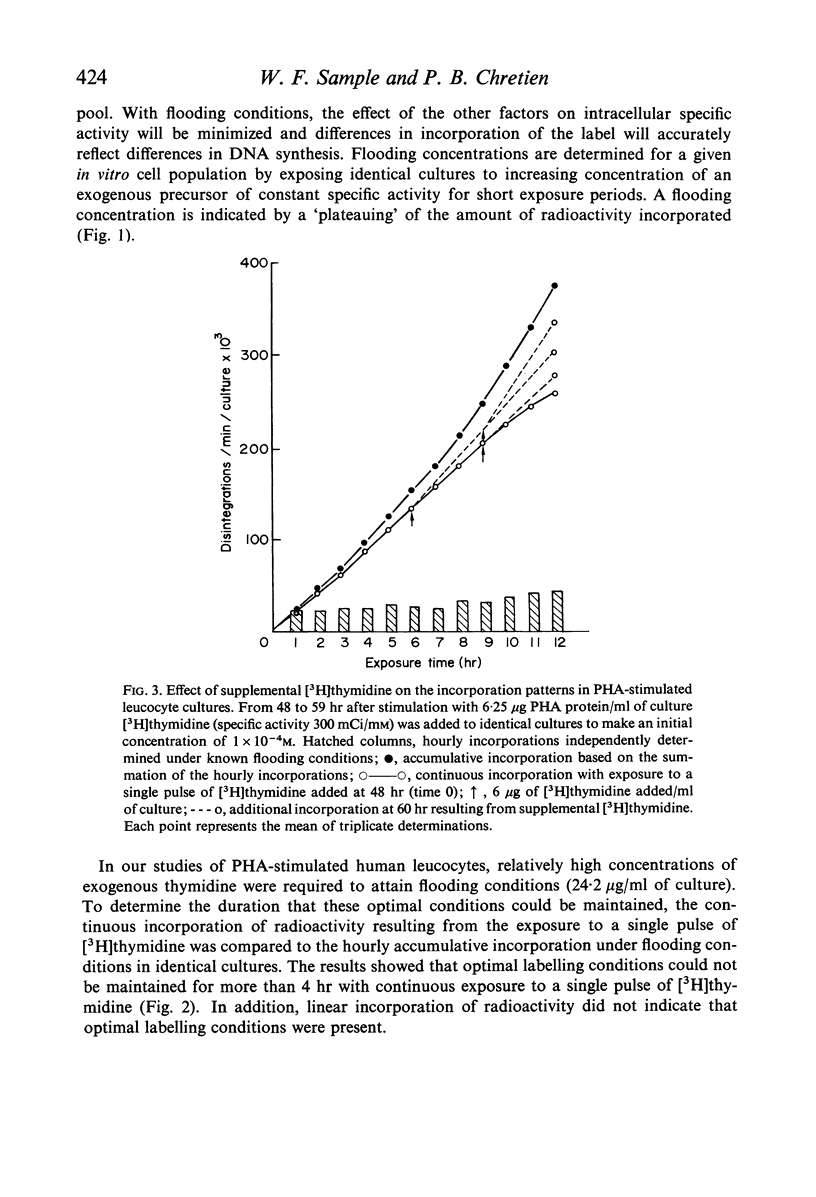
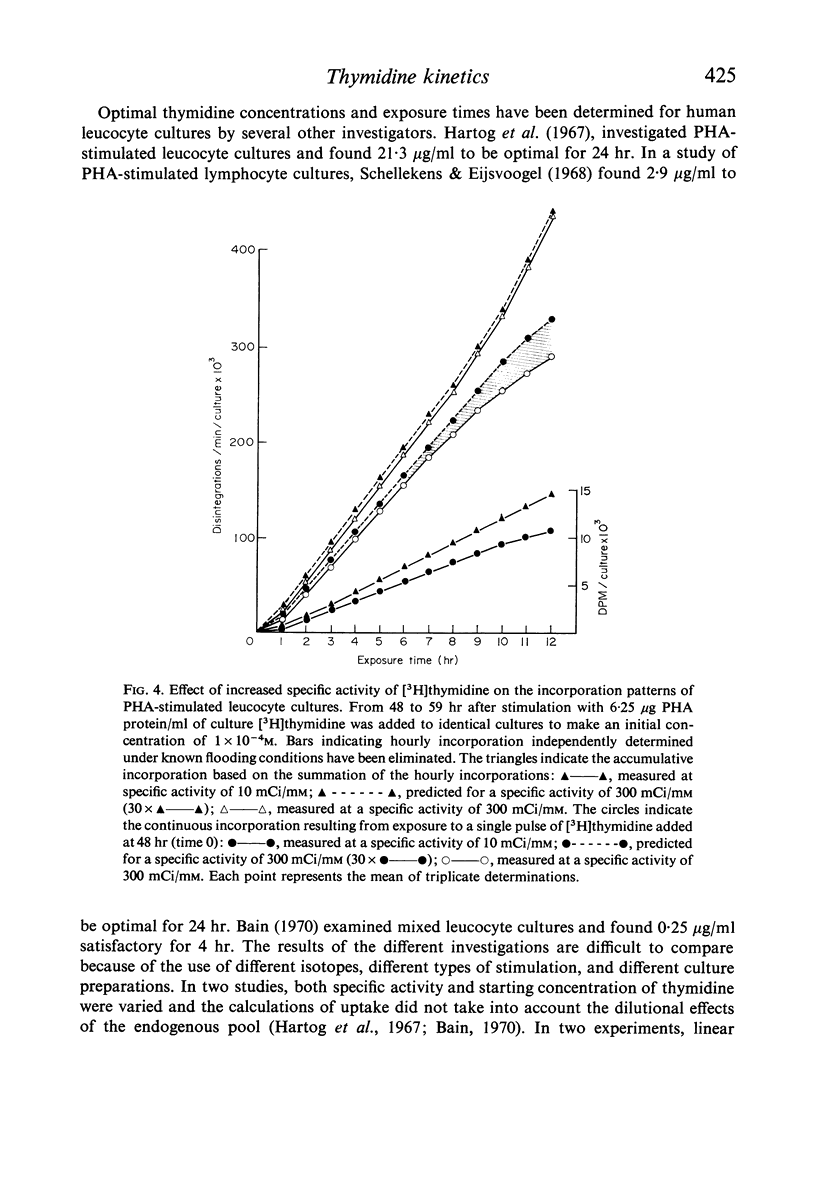


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L. The effect of endogenous pools of thymidylate on the apparent rate of DNA synthesis. Exp Cell Res. 1969 Jul;56(1):55–58. doi: 10.1016/0014-4827(69)90393-0. [DOI] [PubMed] [Google Scholar]
- BOOTSMA D., BUDKE L., VOS O. STUDIES ON SYNCHRONOUS DIVISION OF TISSUE CULTURE CELLS INITIATED BY EXCESS THYMIDINE. Exp Cell Res. 1964 Jan;33:301–309. doi: 10.1016/s0014-4827(64)81035-1. [DOI] [PubMed] [Google Scholar]
- Bain B. Tritiated-thymidine uptake in mixed leucocyte cultures: effect of specific activity and exposure time. Clin Exp Immunol. 1970 Feb;6(2):255–262. [PMC free article] [PubMed] [Google Scholar]
- Boylston A. W., Guttmann R. D., Merrill J. P. A simplified method for the quantitation of in vitro leucocyte culture. Int Arch Allergy Appl Immunol. 1968;34(4):339–344. doi: 10.1159/000230128. [DOI] [PubMed] [Google Scholar]
- COOPER E. H., MILTON J. D. THE INCORPORATION AND DEGRADATION OF PYRIMIDINE DNA PRECURSORS BY HUMAN LEUCOCYTES. Br J Cancer. 1964 Dec;18:701–713. doi: 10.1038/bjc.1964.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREW R. M., PAINTER R. B. Further studies on the clonal growth of HeLa S3 cells treated with tritiated thymidine. Radiat Res. 1962 Mar;16:303–311. [PubMed] [Google Scholar]
- DUTTON R. W., PAGE G. M. THE RESPONSE OF SPLEEN CELLS FROM IMMUNIZED RABBITS TO CROSS-REACTING ANTIGENS, IN AN IN VITRO SYSTEM. Immunology. 1964 Nov;7:665–670. [PMC free article] [PubMed] [Google Scholar]
- Daguillard F., Heiner D. C., Richter M., Rose B. The response of leucocytes of agammaglobulinaemia subjects to phythohaemagglutinin and anti-immunoglobulin antiserum. Clin Exp Immunol. 1969 Feb;4(2):203–211. [PMC free article] [PubMed] [Google Scholar]
- HATCH A., BALAZS T. The use of Cetavlon in a diluent for counting leukocytes in the Coulter electronic counter. A comparison with some currently use diluents. Am J Clin Pathol. 1961 Sep;36:220–223. doi: 10.1093/ajcp/36.3.220. [DOI] [PubMed] [Google Scholar]
- Hartog M., Cline M. J., Grodsky G. M. The response of human leucocyte cultures to stimulation by tuberculin and phytohaemagglutinin measured by the uptake of radioactive thymidine. Clin Exp Immunol. 1967 Mar;2(2):217–228. [PMC free article] [PubMed] [Google Scholar]
- MARSH J. C., PERRY S. THYMIDINE CATABOLISM BY NORMAL AND LEUKEMIC HUMAN LEUKOCYTES. J Clin Invest. 1964 Feb;43:267–278. doi: 10.1172/JCI104911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton J. D., Cooper E. H., Halle-Pannenko O. DLa dégradation de la thymidine par les lymphocytes transformés. Rev Fr Etud Clin Biol. 1965 Apr;10(4):419–422. [PubMed] [Google Scholar]
- Oppenheim J. J. Relationship of in vitro lymphocyte transformation to delayed hypersensitivity in guinea pigs and man. Fed Proc. 1968 Jan-Feb;27(1):21–28. [PubMed] [Google Scholar]
- PAINTER R. B., DREW R. M., HUGHES W. L. Inhibition of HeLa growth by intranuclear tritium. Science. 1958 May 23;127(3308):1244–1245. doi: 10.1126/science.127.3308.1244. [DOI] [PubMed] [Google Scholar]
- Schellekens P. T., Eijsvoogel V. P. Lymphocyte transformation in vitro. I. Tissue culture conditions and quantitative measurements. Clin Exp Immunol. 1968 Jul;3(6):571–584. [PMC free article] [PubMed] [Google Scholar]
- Sorensen S. F., Andersen V., Giese J. A rapid method for quantitation of the incorporation of 3H-thymidine by lymphocytes in vitro. Acta Pathol Microbiol Scand. 1969;75(3):508–511. [PubMed] [Google Scholar]
- Tormey D. C., Kamin R., Fudenberg H. H. Quantitative studies of phytohemagglutinin-induced DNA and RNA synthesis in normal and agammaglobulinemic leukocytes. J Exp Med. 1967 May 1;125(5):863–872. doi: 10.1084/jem.125.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XEROS N. Deoxyriboside control and synchronization of mitosis. Nature. 1962 May 19;194:682–683. doi: 10.1038/194682a0. [DOI] [PubMed] [Google Scholar]


