Abstract
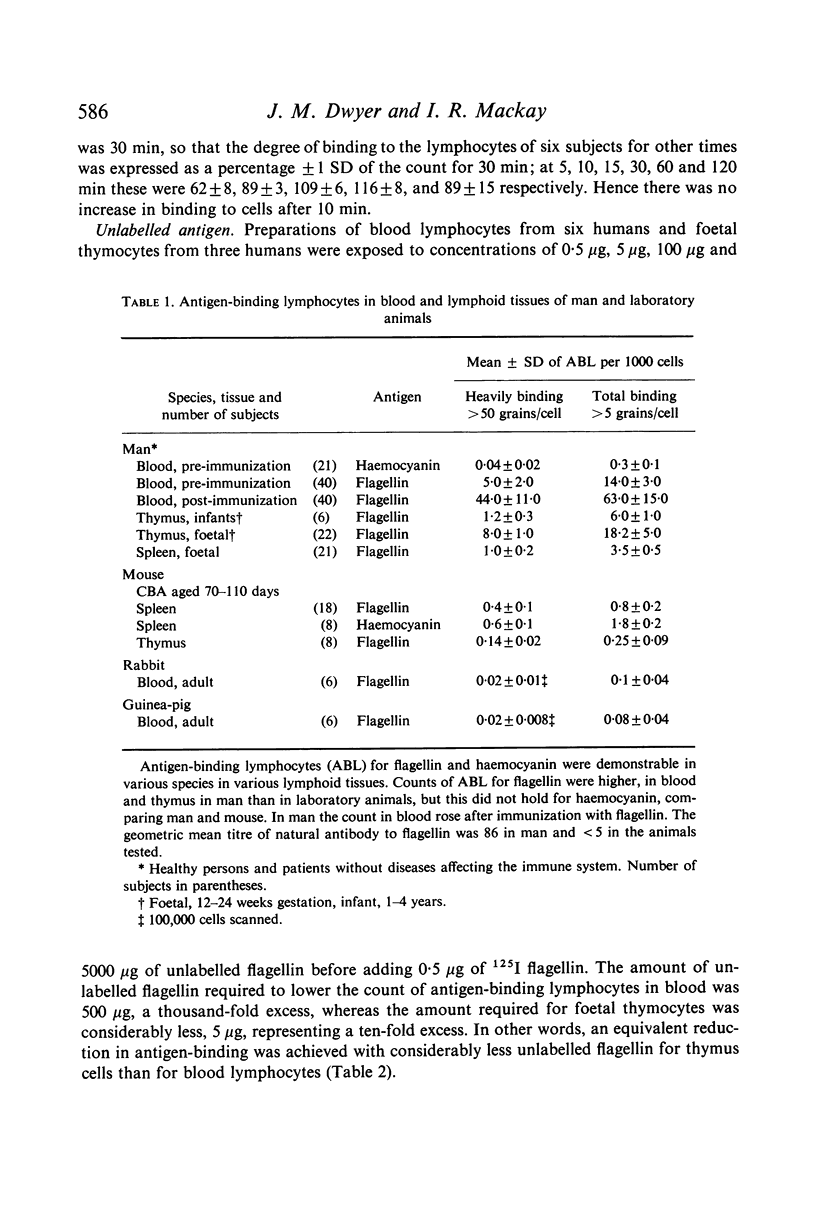
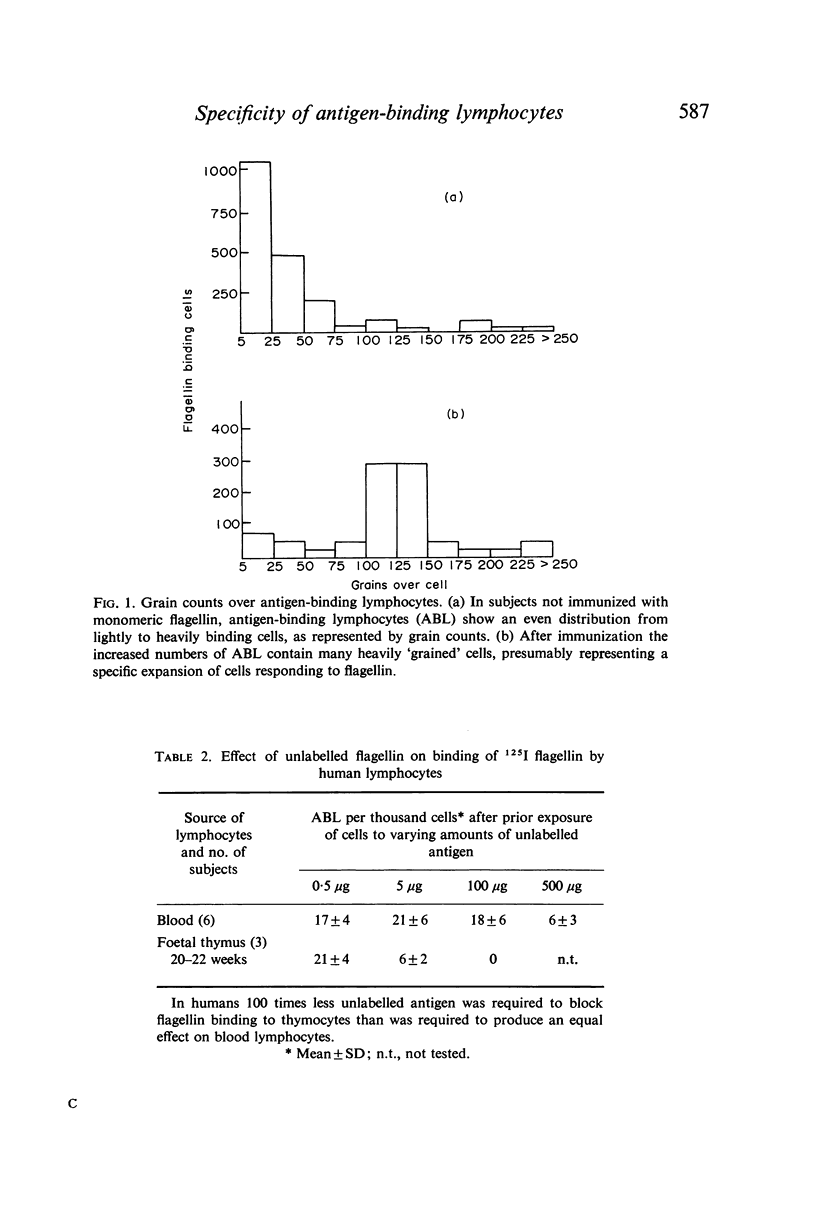
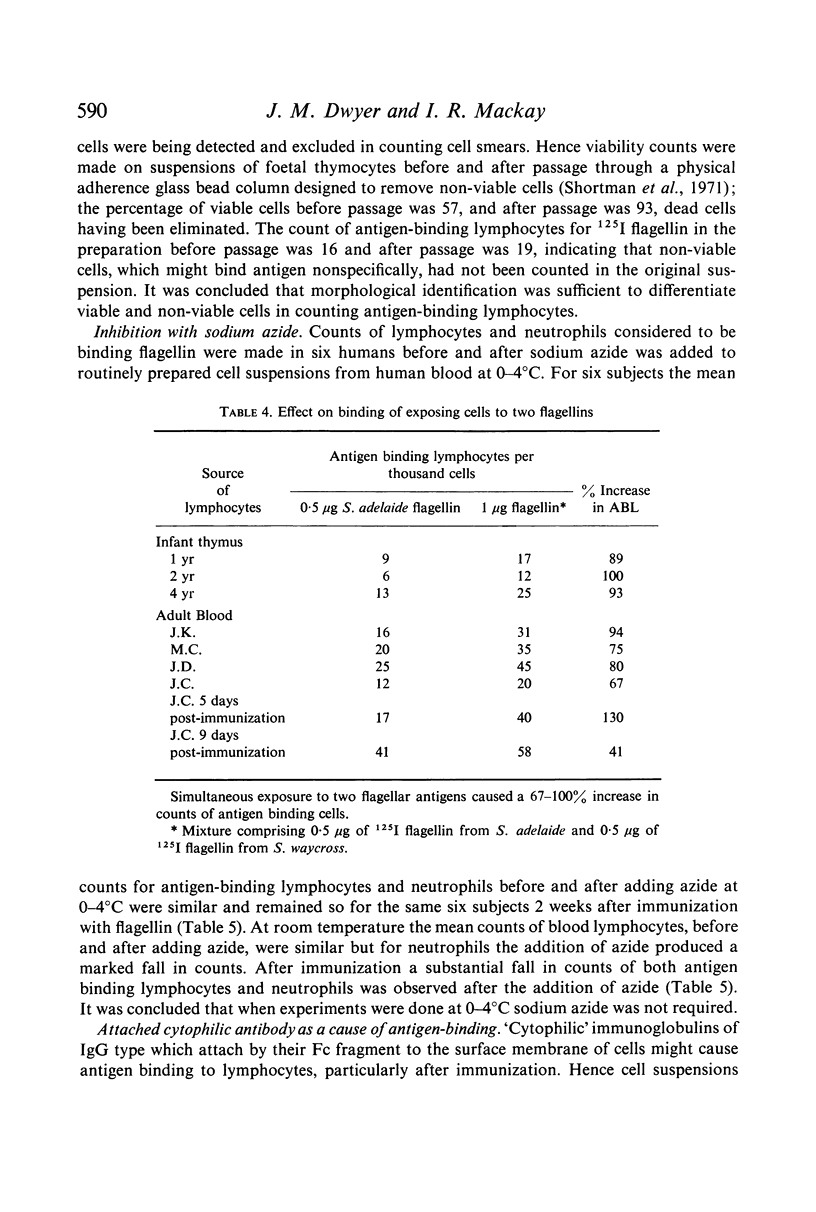
Antigen-binding lymphocytes were recognized by their reaction with radioiodine labelled antigens such as flagellin and haemocyanin. Counts varied according to the antigen and species studied. For flagellin, counts in human blood of antigen-binding lymphocytes (mean ± 1 SD per 1000 lymphocytes) were 19·0±3·0, and in foetal thymus 18·2±5·0 and spleen 3·5±0·5. Results depended on contact time of cells with antigen, concentration of antigen, autoradiographic exposure, presence of natural antibody and antibody levels after immunization. Antigen-binding lymphocytes in blood were not antibody-producing cells. The specificity of the antigen-binding reaction was shown by exposing lymphocytes to 0·5 μg of two antigenically distinct flagellins; there was a 67–100% increase in the counts in contrast to the 20–45% increase on doubling the dose (0·5 μg to 1 μg) of flagellin from Salmonella adelaide. Cytophilic antibody as the cause of antigen binding was excluded.
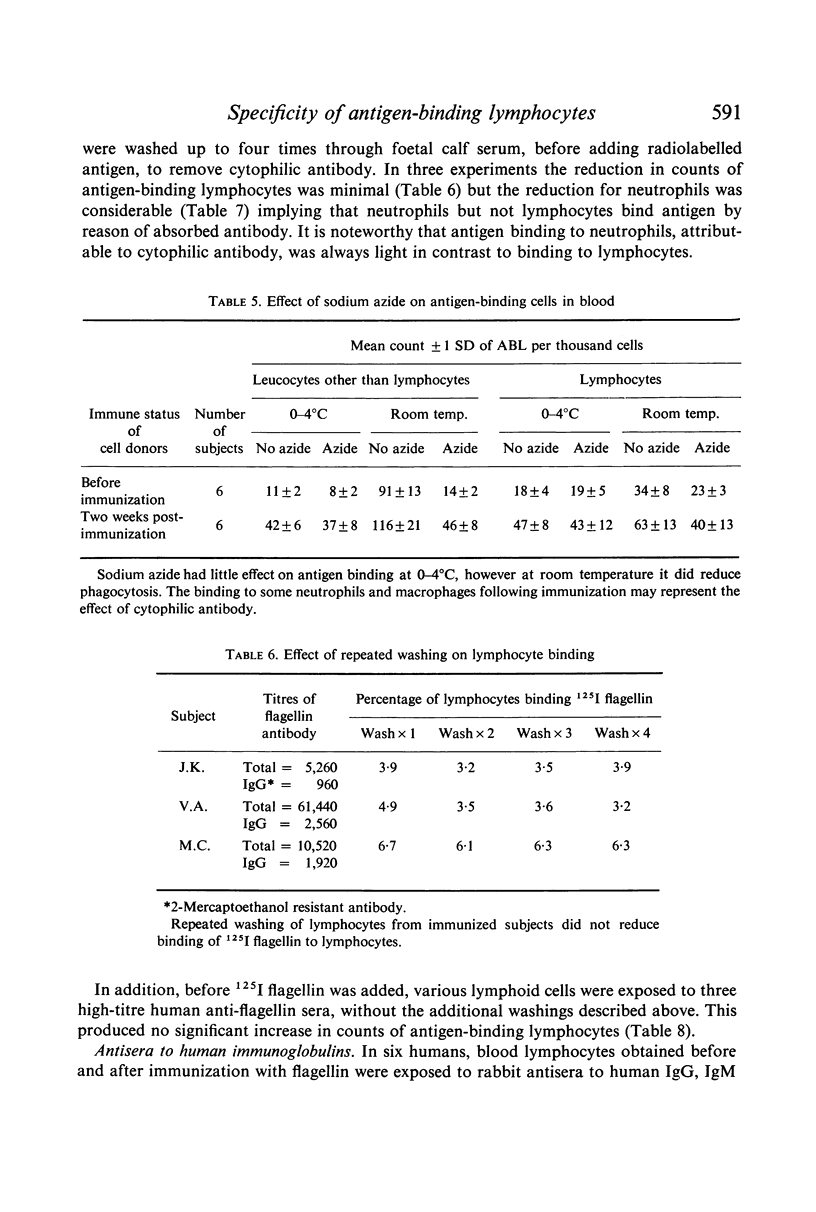
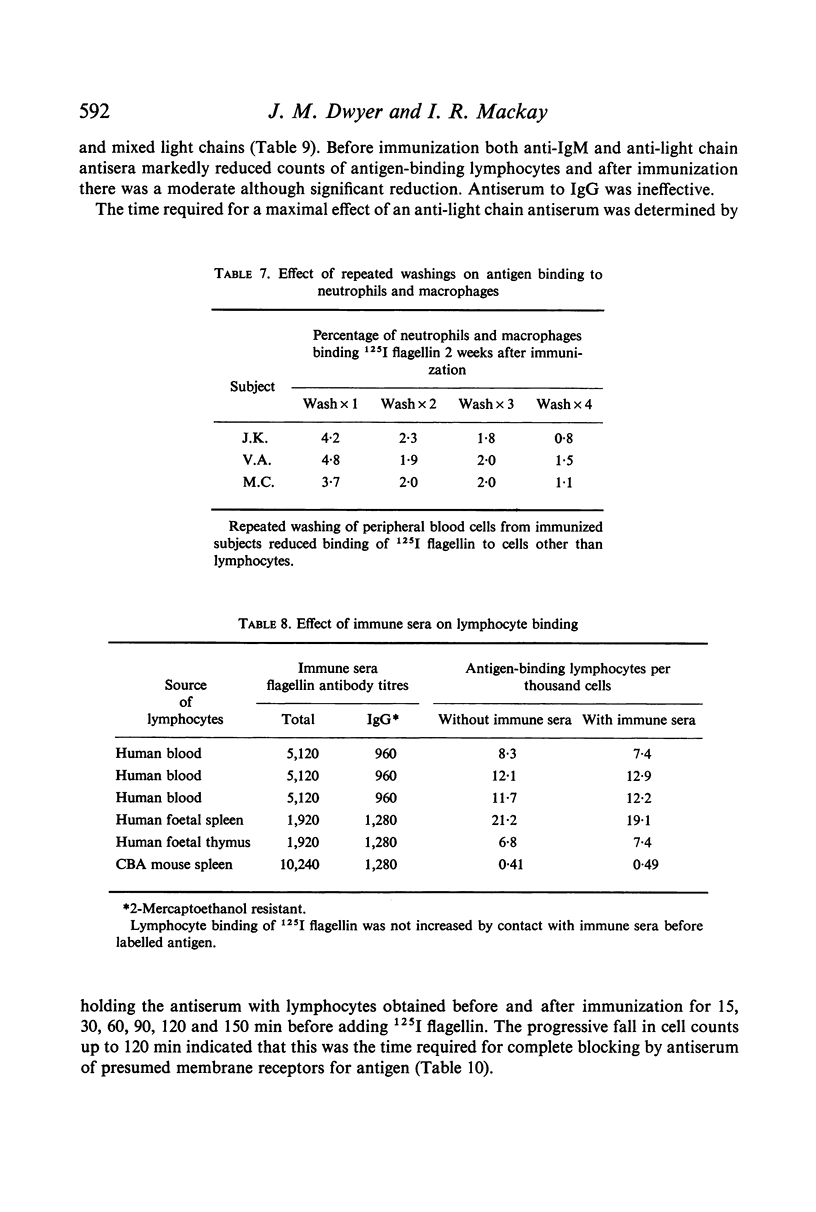
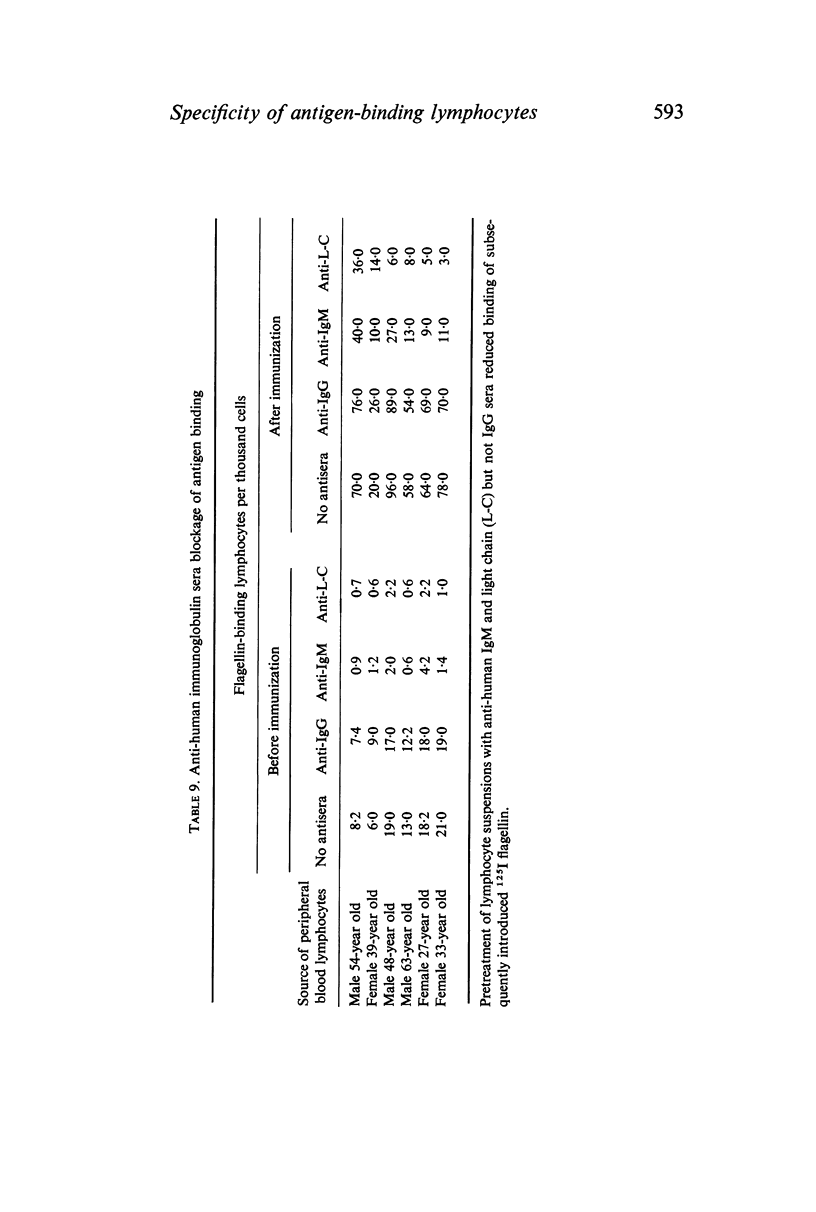
The binding of flagellin to lymphocytes was prevented by anti-human IgM and light chain antisera, but not anti-human IgG sera. The binding of labelled flagellin was prevented by unlabelled flagellin but 100 times more was needed for blood lymphocytes than thymocytes. It is inferred that thymocytes, T cells, have considerably fewer receptors than most β lymphocytes detectable in blood.
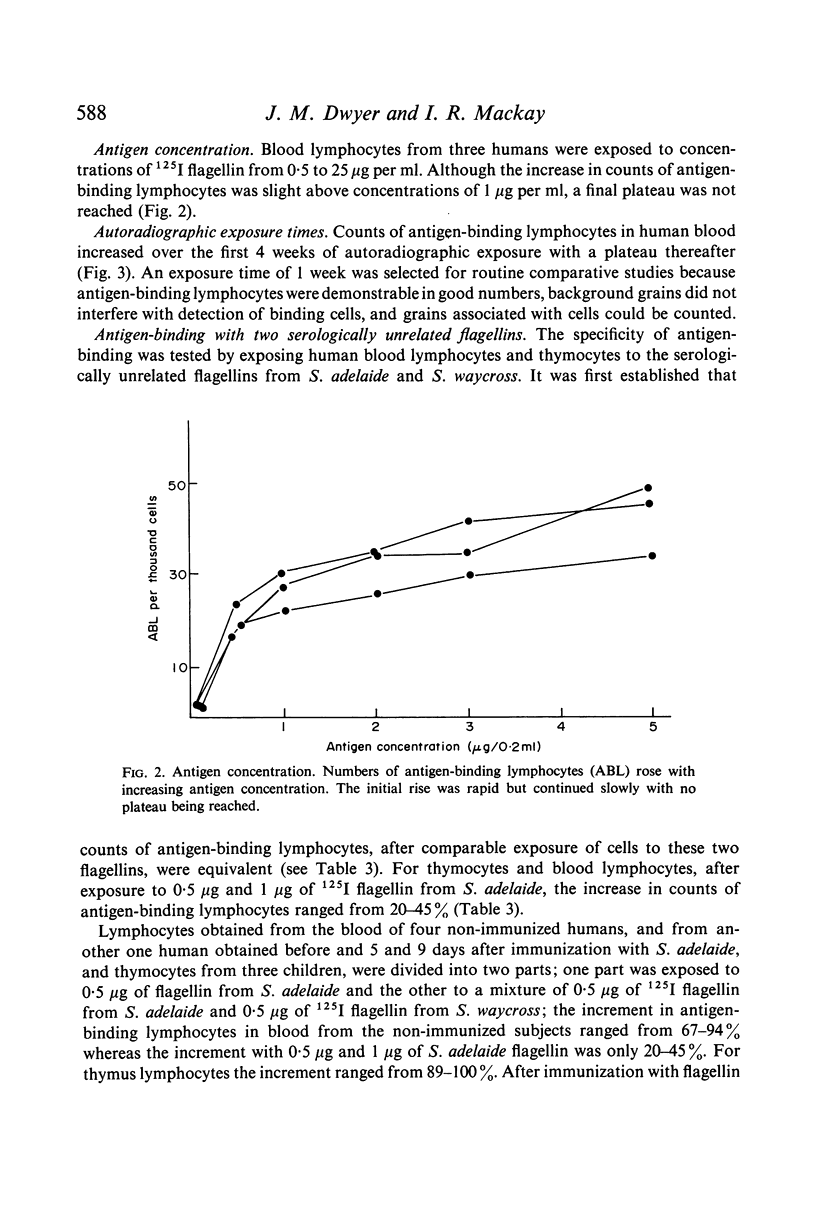
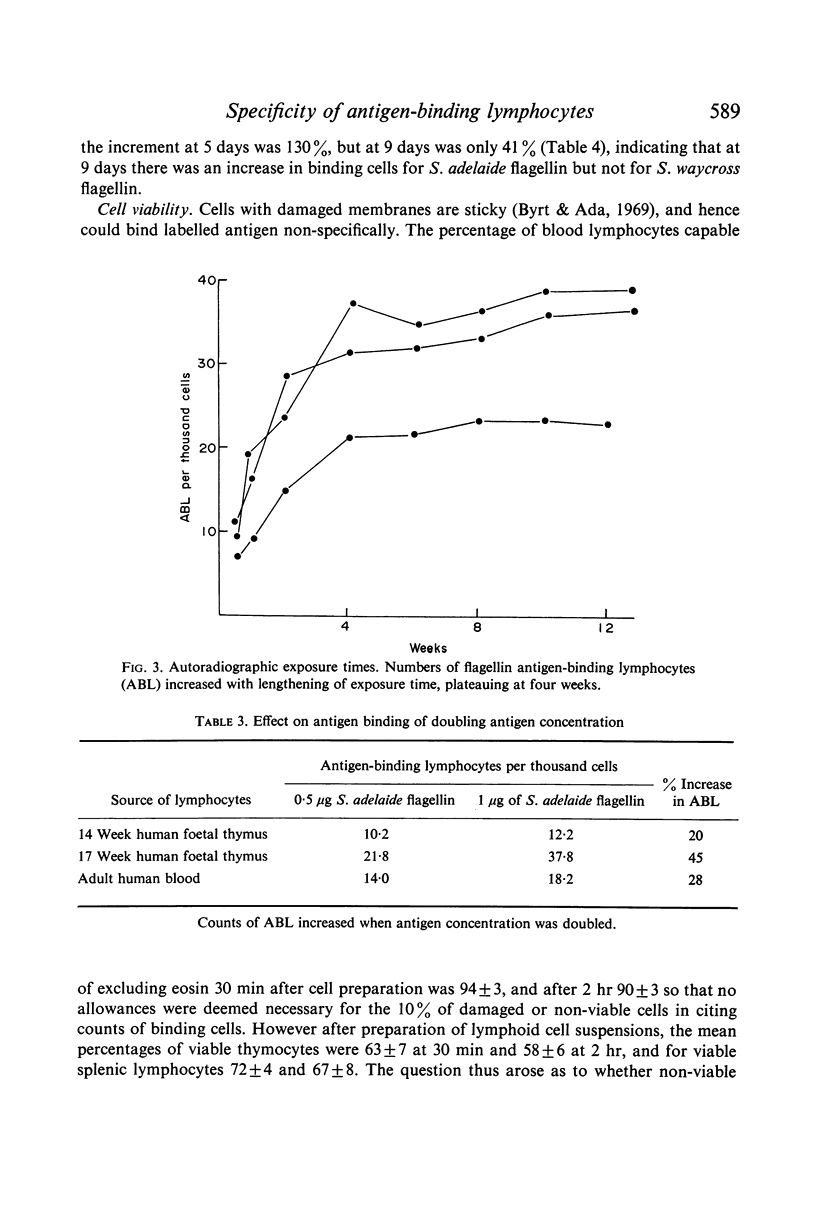
Using standardized conditions, radiolabelled antigen binding provides a reproducible, immunologically specific and flexible technique allowing study of the nature and role of antigen-binding cells and cell surface receptors.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., NOSSAL G. J., PYE J., ABBOT A. ANTIGENS IN IMMUNITY. I. PREPARATION AND PROPERTIES OF FLAGELLAR ANTIGENS FROM SALMONELLA ADELAIDE. Aust J Exp Biol Med Sci. 1964 Jun;42:267–282. [PubMed] [Google Scholar]
- Ada G. L. Antigen binding cells in tolerance and immunity. Transplant Rev. 1970;5:105–129. doi: 10.1111/j.1600-065x.1970.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Ada G. L., Byrt P. Specific inactivation of antigen-reactive cells with 125I-labelled antigen. Nature. 1969 Jun 28;222(5200):1291–1292. doi: 10.1038/2221291a0. [DOI] [PubMed] [Google Scholar]
- Byrt P., Ada G. L. An in vitro reaction between labelled flagellin or haemocyanin and lymphocyte-like cells from normal animals. Immunology. 1969 Oct;17(4):503–516. [PMC free article] [PubMed] [Google Scholar]
- Diener E. A new method for the enumeration of single antibody-producing cells. J Immunol. 1968 May;100(5):1062–1070. [PubMed] [Google Scholar]
- Dwyer J. M., Mackay I. R. Antigen-binding lymphocytes in human blood. Lancet. 1970 Jan 24;1(7639):164–167. doi: 10.1016/s0140-6736(70)90406-x. [DOI] [PubMed] [Google Scholar]
- Dwyer J. M., Warner N. L. Antigen binding cells in embryonic chicken bursa and thymus. Nat New Biol. 1971 Feb 17;229(7):210–211. doi: 10.1038/newbio229210a0. [DOI] [PubMed] [Google Scholar]
- Greaves M. F. Biological effects of anti-immunoglobulins: evidence for immunoglobulin receptors on 'T' and 'B' lymphocytes. Transplant Rev. 1970;5:45–75. doi: 10.1111/j.1600-065x.1970.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Hood L., Talmage D. W. Mechanism of antibody diversity: germ line basis for variability. Science. 1970 Apr 17;168(3929):325–334. doi: 10.1126/science.168.3929.325. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Naor D., Sulitzeanu D. Affinity of radioiodinated bovine serum albumin for lymphoid cells. Binding of I-125-BSA to lymphoid cells of immune mice. Isr J Med Sci. 1969 Mar-Apr;5(2):217–229. [PubMed] [Google Scholar]
- Naor D., Sulitzneau D. Binding of radioiodinated bovine serum albumin to mouse spleen cells. Nature. 1967 May 13;214(5089):687–688. doi: 10.1038/214687a0. [DOI] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley M. J. "Natural" antibody in man to flagellar antigens of Salmonella adelaide. Aust J Exp Biol Med Sci. 1970 Apr;48(2):249–252. doi: 10.1038/icb.1970.26. [DOI] [PubMed] [Google Scholar]
- Rowley M. J., Mackay I. R. Measurement of antibody-producing capacity in man. I. The normal response to flagellin from Salmonella adelaide. Clin Exp Immunol. 1969 Oct;5(4):407–418. [PMC free article] [PubMed] [Google Scholar]
- Wigzell H. Specific fractionation of immunocompetent cells. Transplant Rev. 1970;5:76–104. doi: 10.1111/j.1600-065x.1970.tb00357.x. [DOI] [PubMed] [Google Scholar]
- Wistar R., Jr Serum antibody to Salmonella flagellar antigens. I. Methods of antibody assay. Aust J Exp Biol Med Sci. 1968 Dec;46(6):769–777. doi: 10.1038/icb.1968.183. [DOI] [PubMed] [Google Scholar]


