Abstract
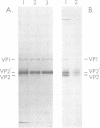
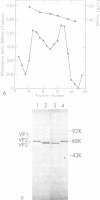
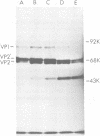
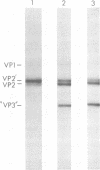
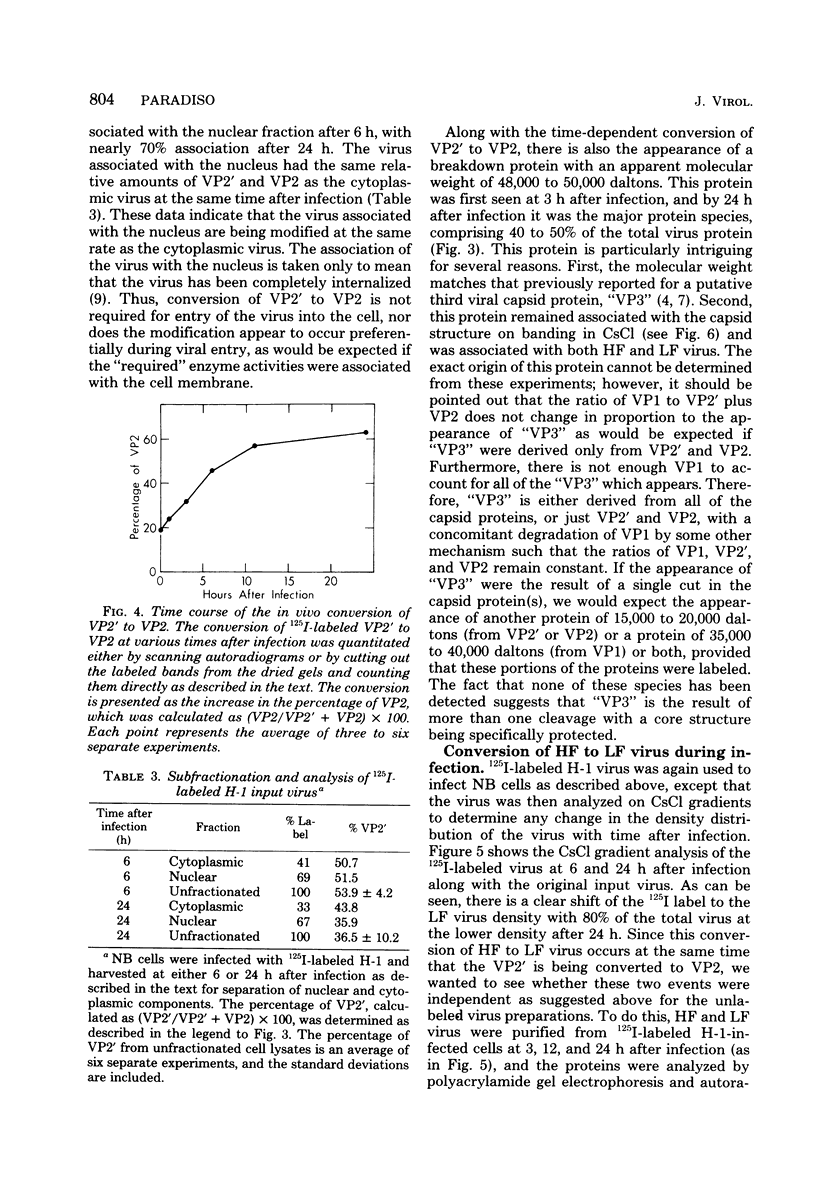
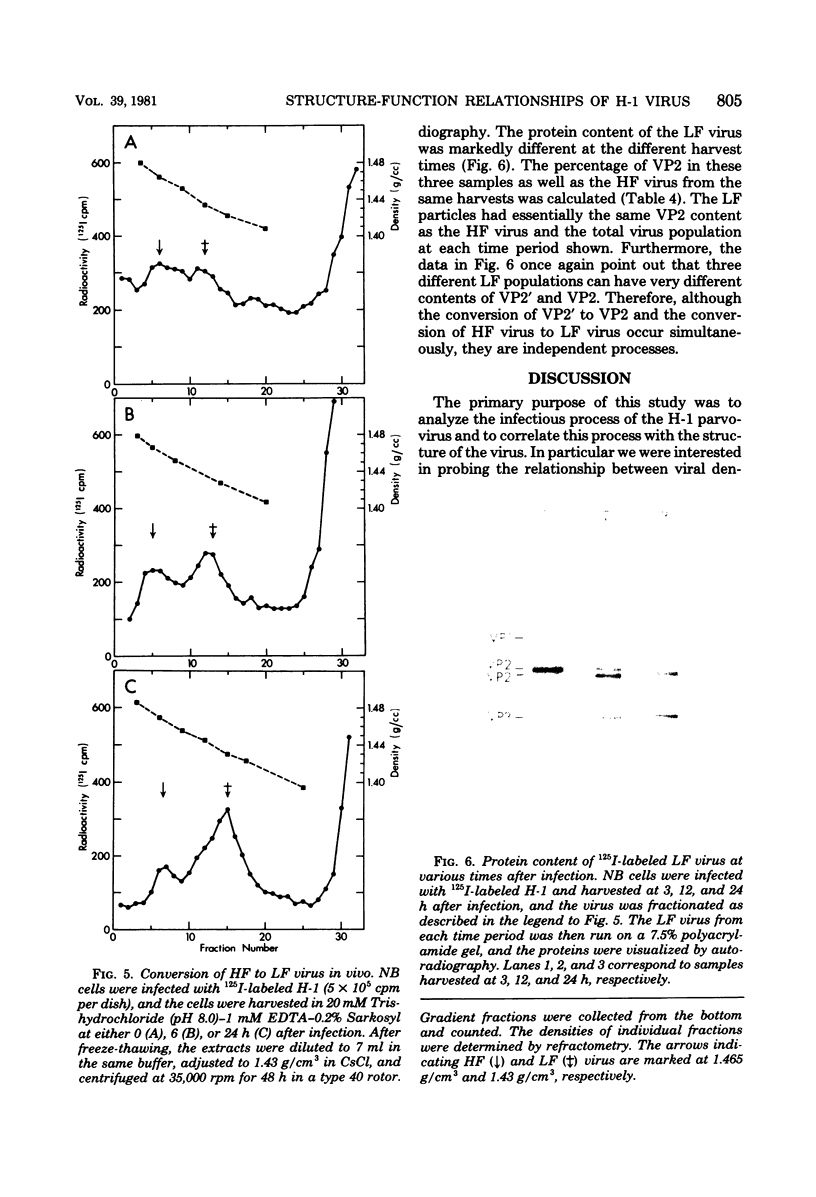
The infectious particles of the parvovirus H-1 were characterized with respect to protein content, density in CsCl, and specific infectivity. Heavy-full and light-full particles were purified from infected simian virus 40-transformed newborn human kidney (NB) cells and from simian virus 40-transformed hamster kidney (THK) cells. Analysis of the protein content of these particles demonstrated that the ratio of viral protein VP2' to VP2 was the same in heavy-full and light-full particles derived from the same cell line, but differed significantly between the two hosts. However, the infectivity of the particles from each cell line was the same for all four viral species.. Also, in vitro conversion of VP2' to VP2 did not enhance the particle infectivity of either heavy-full or light-full virus. When the fate of input virus was studied with 125I-labeled H-1, the conversion of VP2' to VP2 occurred in a time-dependent manner up to 24 h postinfection. Simultaneous with the proteolytic cleavage, there was a shift in the density of the heavy-full virus to the light-full density. However, protein analysis of the 125I-labeled light-full virus at various times postinfection indicated that they were not enriched in VP2 when compared with heavy-full virus or the total virus population. Thus, the cleavage of VP2' to VP2 is not responsible for the shift in density from heavy-full to light-full virus, and although these events might be required for infection they appear not to be interdependent.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clinton G. M., Hayashi M. The parovivirus MVM: particles with altered structural proteins. Virology. 1975 Jul;66(1):261–261. doi: 10.1016/0042-6822(75)90196-8. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Hayashi M. The parvovirus MVM: a comparison of heavy and light particle infectivity and their density conversion in vitro. Virology. 1976 Oct 1;74(1):57–63. doi: 10.1016/0042-6822(76)90127-6. [DOI] [PubMed] [Google Scholar]
- Handa H., Shimojo H. Isolation of the viral DNA replication complex from adeno-associated virus type 1-infected cells. J Virol. 1977 Nov;24(2):444–450. doi: 10.1128/jvi.24.2.444-450.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsvik J. R., Gierthy J. F., Rhode S. L., 3rd Replication process of the parvovirus H-1. IV. H-1-specific proteins synthesized in synchronized human NB kidney cells. J Virol. 1974 Dec;14(6):1600–1603. doi: 10.1128/jvi.14.6.1600-1603.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsvik J. R., Hopkins M. S., Ellem K. A. Subfractionation of CsCl-purified H-1 parvovirus on metrizamide gradients. Virology. 1979 Jul 30;96(2):646–651. doi: 10.1016/0042-6822(79)90122-3. [DOI] [PubMed] [Google Scholar]
- Kongsvik J. R., Toolan H. W. Capsid components of the parvovirus H-1. Proc Soc Exp Biol Med. 1972 Apr;139(4):1202–1205. doi: 10.3181/00379727-139-36329. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linser P., Bruning H., Armentrout R. W. Specific binding sites for a parvovirus, minute virus of mice, on cultured mouse cells. J Virol. 1977 Oct;24(1):211–221. doi: 10.1128/jvi.24.1.211-221.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Replication process of the parvovirus H-1. VII. Electron microscopy of replicative-form DNA synthesis. J Virol. 1977 Feb;21(2):713–723. doi: 10.1128/jvi.21.2.713-723.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- Usategui-Gomez M., Toolan H. W., Ledinko N., al-Lami F., Hopkins M. S. Single-stranded DNA from the Parvovirus, H-1. Virology. 1969 Nov;39(3):617–621. doi: 10.1016/0042-6822(69)90117-2. [DOI] [PubMed] [Google Scholar]