Abstract
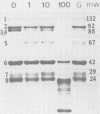
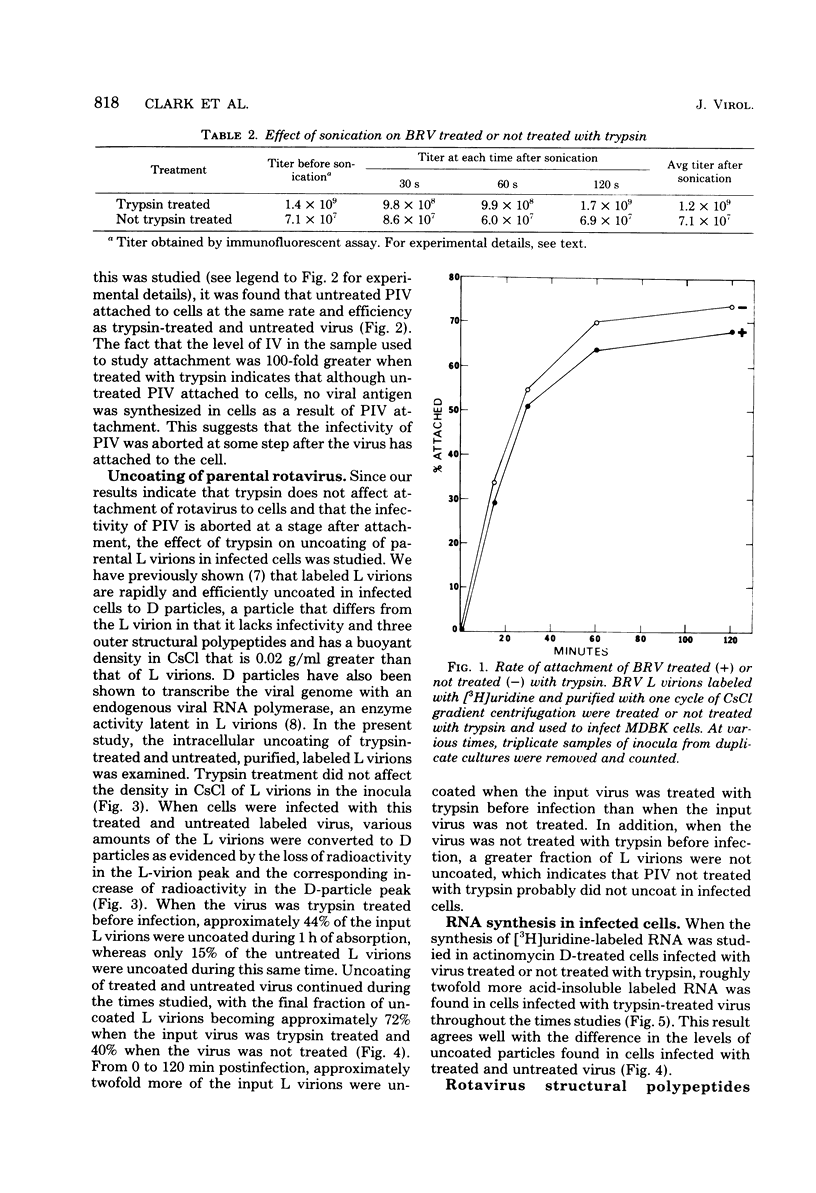
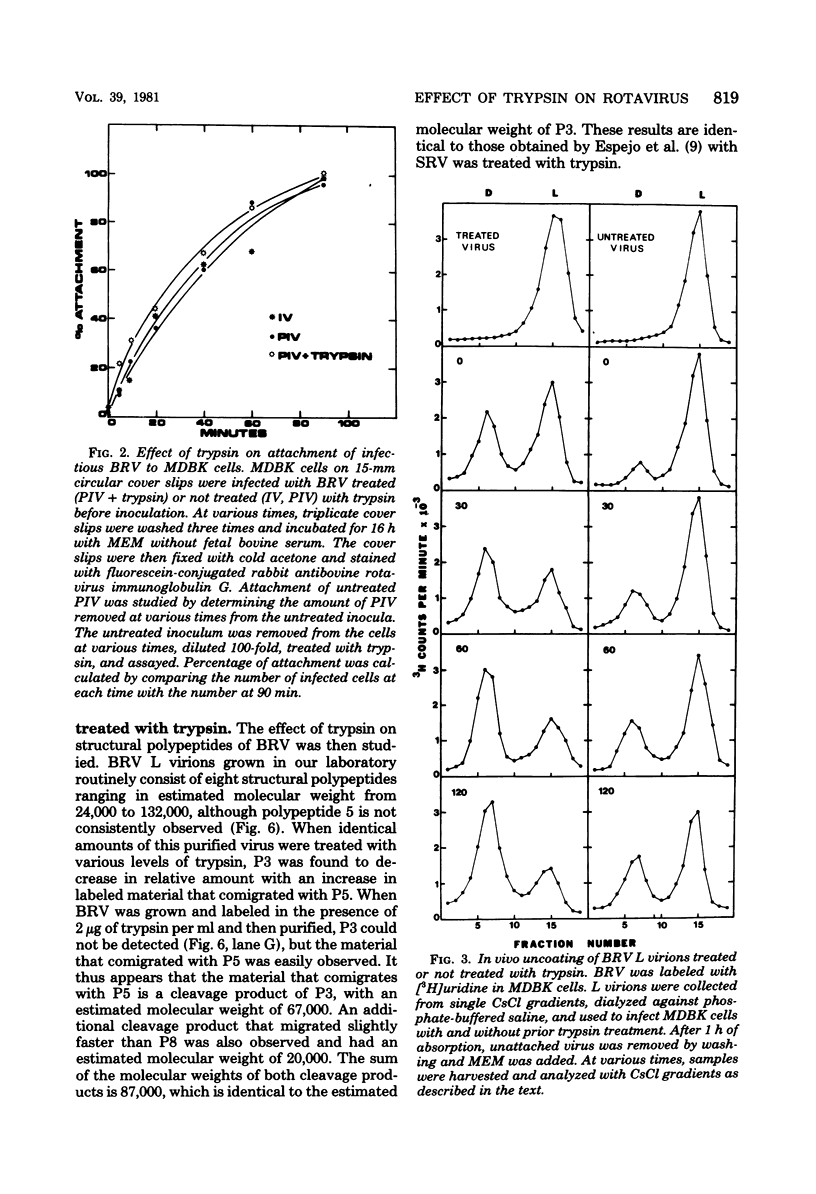
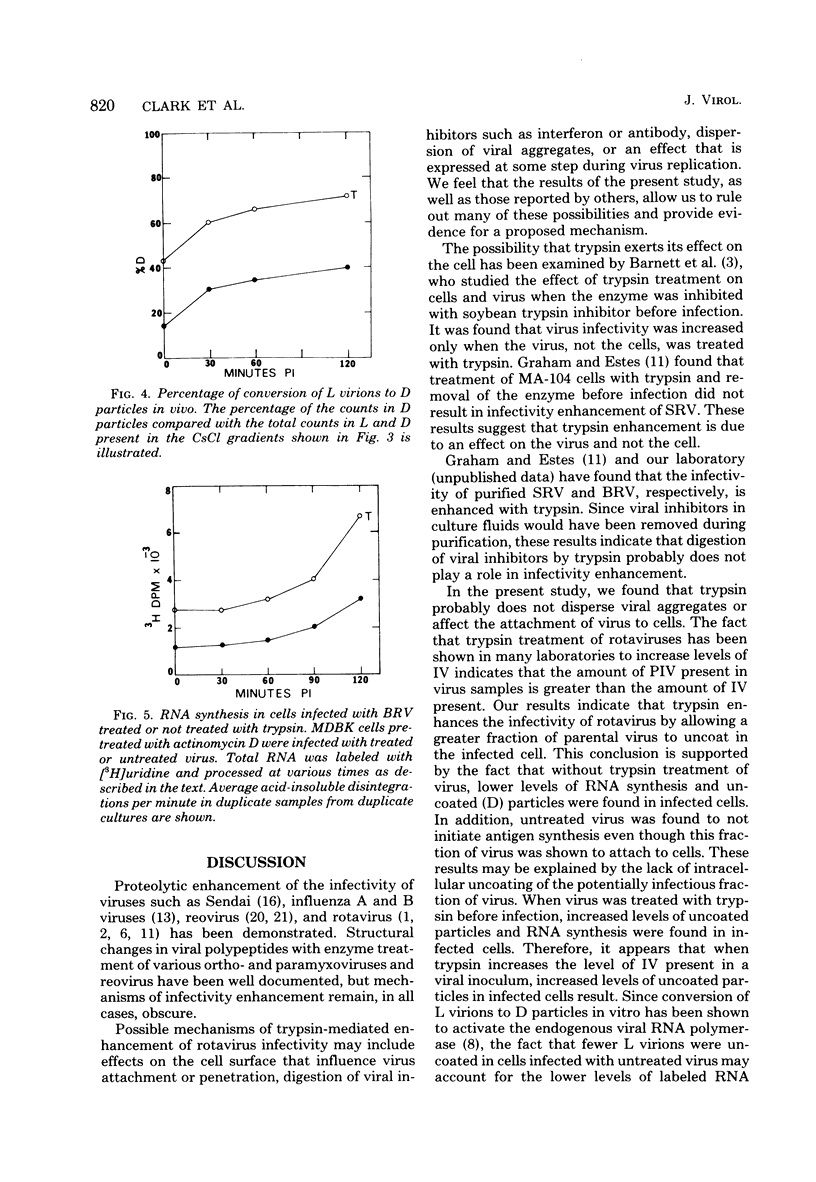
The infectivity of most rotaviruses is enhanced by treatment with trypsin. We studied the mechanism of enhancement of examining the effect of trypsin on rotavirus infectivity, aggregation, early interactions with host cells, and structure. The results indicated that trypsin does not increase levels of infectious virus by dispersion of aggregates or affect the efficiency or rate of attachment of virus to cells. A fraction of virus that was not infections without trypsin treatment was found to attach to cells, but did not initiate antigen synthesis. When cells were infected with labeled, purified virus, increased levels of uncoated particles were found in cells infected with trypsin-treated virus. Infection of cells with trypsin-treated virus also led to greater levels of RNA synthesis early in the infection. The results suggest that trypsin converts a noninfectious fraction of virus into infectious virus by allowing this fraction to uncoat in the infected cell. Trypsin was found to cleave an 88,000-dalton structural polypeptide of bovine rotavirus generating 67,000- and 20,000-dalton cleavage products.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D., Hall T., Banatvala J. E., Totterdell B. M., Chrystie I. L. The effect of trypsin on the growth of rotavirus. J Gen Virol. 1978 Jul;40(1):213–218. doi: 10.1099/0022-1317-40-1-213. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Mohammed K., Spence L., Fauvel M., Petro R. Rotavirus isolation and cultivation in the presence of trypsin. J Clin Microbiol. 1977 Dec;6(6):610–617. doi: 10.1128/jcm.6.6.610-617.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett B. B., Spendlove R. S., Clark M. L. Effect of enzymes on rotavirus infectivity. J Clin Microbiol. 1979 Jul;10(1):111–113. doi: 10.1128/jcm.10.1.111-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett B. B., Spendlove R. S., Peterson M. W., Hsu L. Y., LaSalle V. A., Egbert L. N. Immunofluorescent cell assay of neonatal calf diarrhea virus. Can J Comp Med. 1975 Oct;39(4):462–465. [PMC free article] [PubMed] [Google Scholar]
- Bryden A. S., Davies H. A., Thouless M. E., Flewitt T. H. Diagnosis of rotavirus infection by cell culture. J Med Microbiol. 1977 Feb;10(1):121–125. doi: 10.1099/00222615-10-1-121. [DOI] [PubMed] [Google Scholar]
- Clark S. M., Barnett B. B., Spendlove R. S. Production of high-titer bovine rotavirus with trypsin. J Clin Microbiol. 1979 Mar;9(3):413–417. doi: 10.1128/jcm.9.3.413-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. M., Spendlove R. S., Barnett B. B. Role of two particle types in bovine rotavirus morphogenesis. J Virol. 1980 Apr;34(1):272–276. doi: 10.1128/jvi.34.1.272-276.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., López S., Arias C. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. J Virol. 1981 Jan;37(1):156–160. doi: 10.1128/jvi.37.1.156-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Estes M. K. Proteolytic enhancement of rotavirus infectivity: biology mechanism. Virology. 1980 Mar;101(2):432–439. doi: 10.1016/0042-6822(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Inouye S., Kono R. Plaque assay of neonatal calf diarrhea virus and the neutralizing antibody in human sera. J Clin Microbiol. 1977 Jan;5(1):1–4. doi: 10.1128/jcm.5.1.1-4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosai R. B., Gardner P. S., Almeida J. D., Greenaway M. A. A simple immunofluorescent technique for the detection of human rotavirus. J Med Virol. 1979;3(3):189–194. doi: 10.1002/jmv.1890030304. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Schoub B. D., Kalica A. R., Greenberg H. B., Bertran D. M., Sereno M. M., Wyatt R. G., Chanock R. M., Kapikian A. Z. Enhancement of antigen incorporation and infectivity of cell cultures by human rotavirus. J Clin Microbiol. 1979 Apr;9(4):488–492. doi: 10.1128/jcm.9.4.488-492.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. M., Estes M. K., Graham D. Y., Gerba C. P. A plaque assay for the simian rotavirus SAII. J Gen Virol. 1979 Jun;43(3):513–519. doi: 10.1099/0022-1317-43-3-513. [DOI] [PubMed] [Google Scholar]
- Spendlove R. S., McClain M. E., Lennette E. H. Enhancement of reovirus infectivity by extracellular removal or alteration of the virus capsid by proteolytic enzymes. J Gen Virol. 1970 Aug;8(2):83–94. doi: 10.1099/0022-1317-8-2-83. [DOI] [PubMed] [Google Scholar]
- Theil K. W., Bohl E. H., Agnes A. G. Cell culture propagation of porcine rotavirus (reovirus-like agent). Am J Vet Res. 1977 Nov;38(11):1765–1768. [PubMed] [Google Scholar]