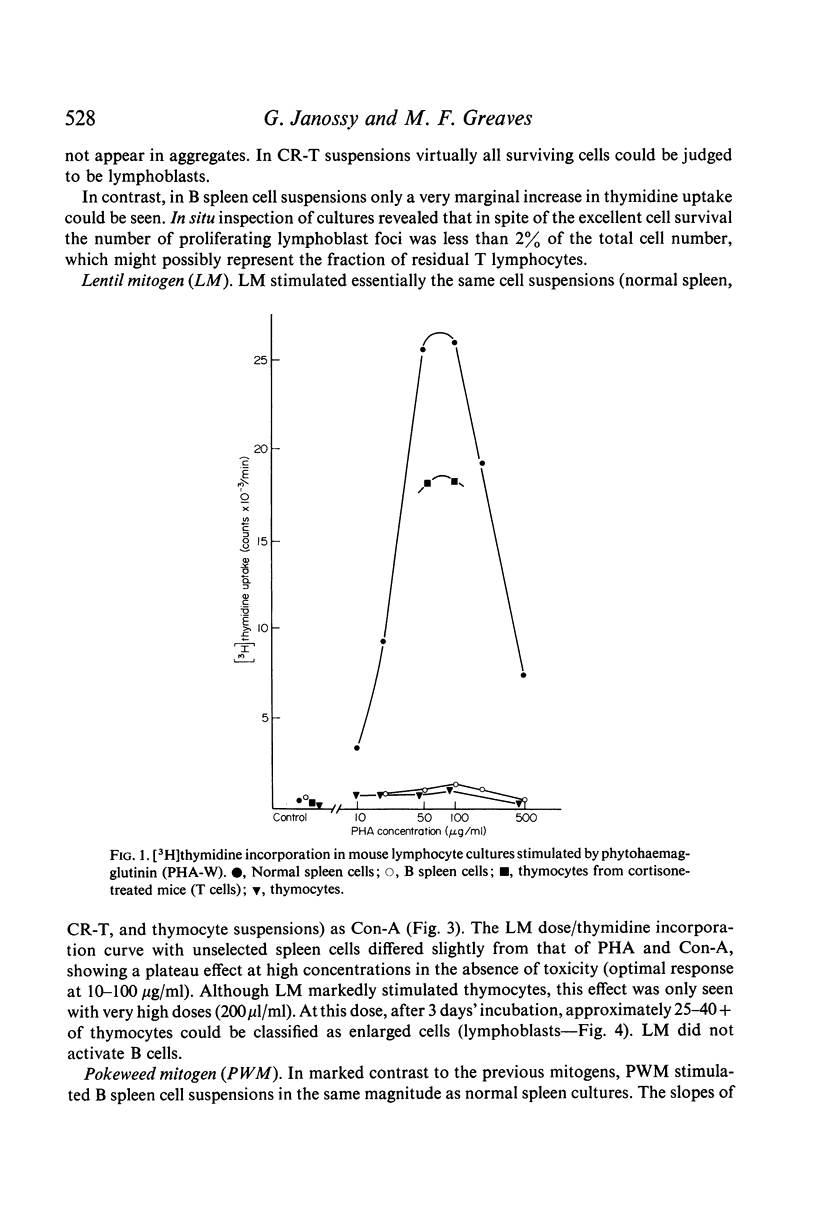
Abstract
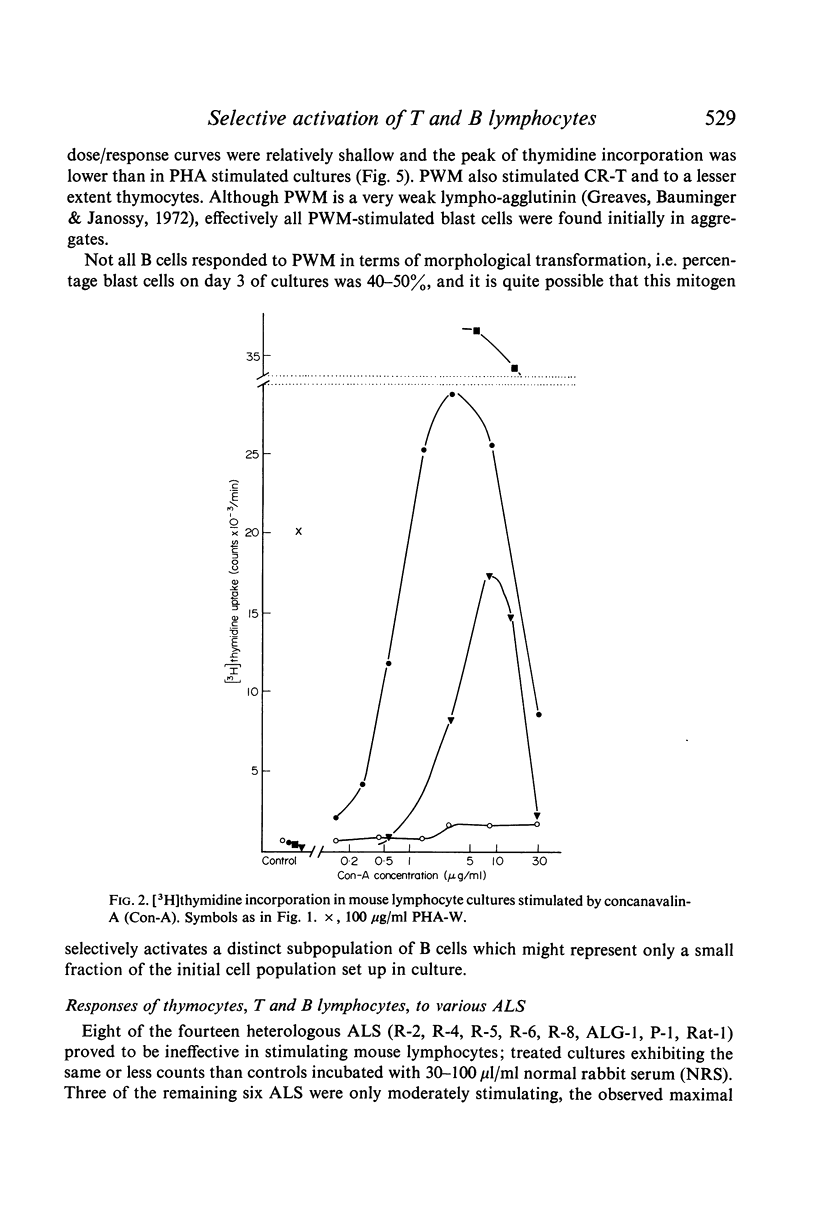
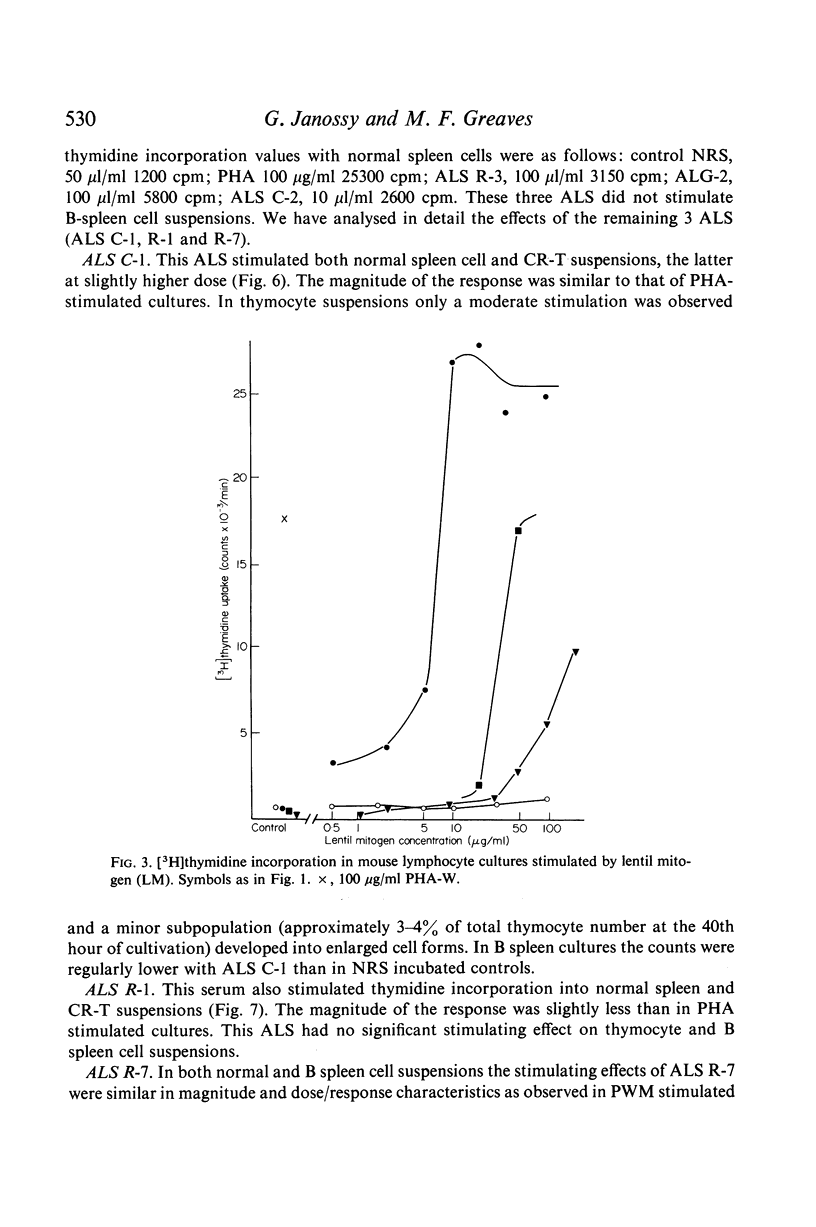
The mitogenic selectivity of four phytomitogens, phytohaemagglutinin (PHA), concanavalin (ConA), lentil mitogen (LM) and pokeweed mitogen (PWM), and a series of heterologous antilymphocyte sera (ALS) for mouse T and B lymphocytes has been investigated using thymidine uptake as a measure of proliferative activity.
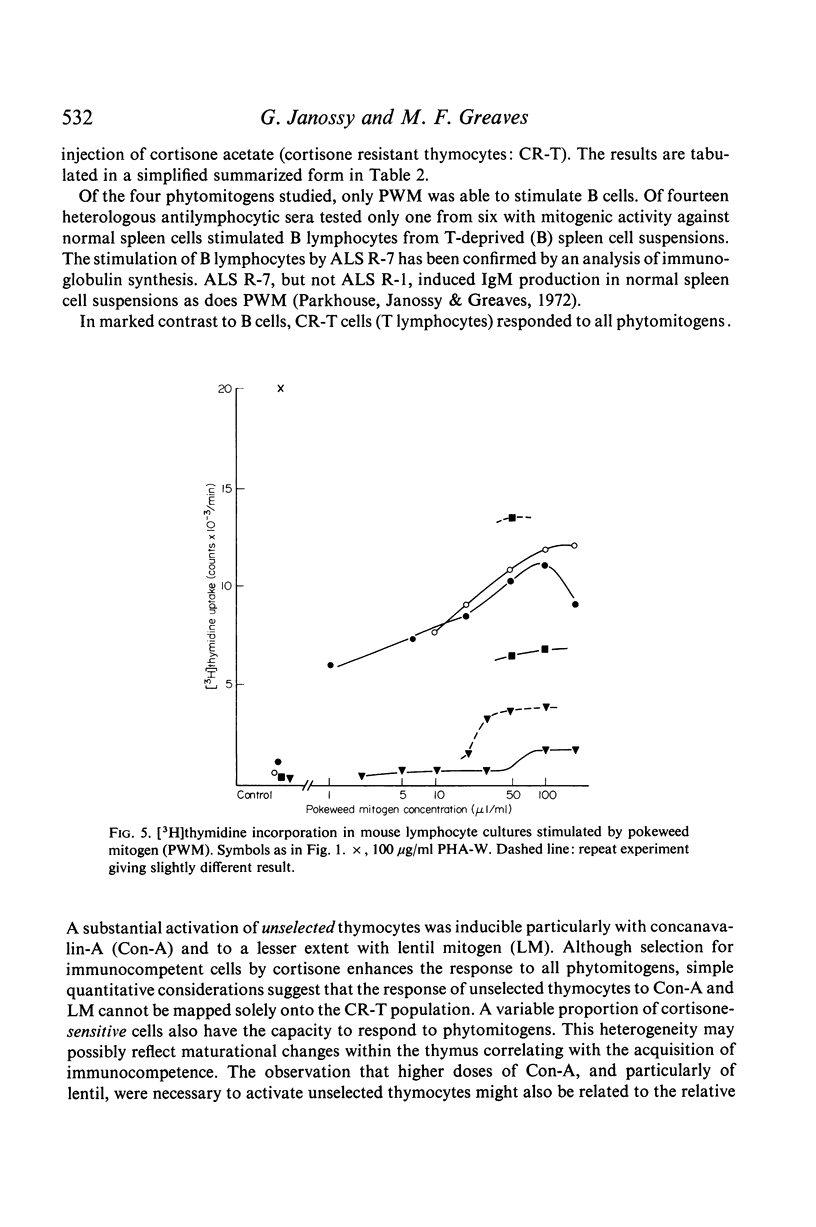
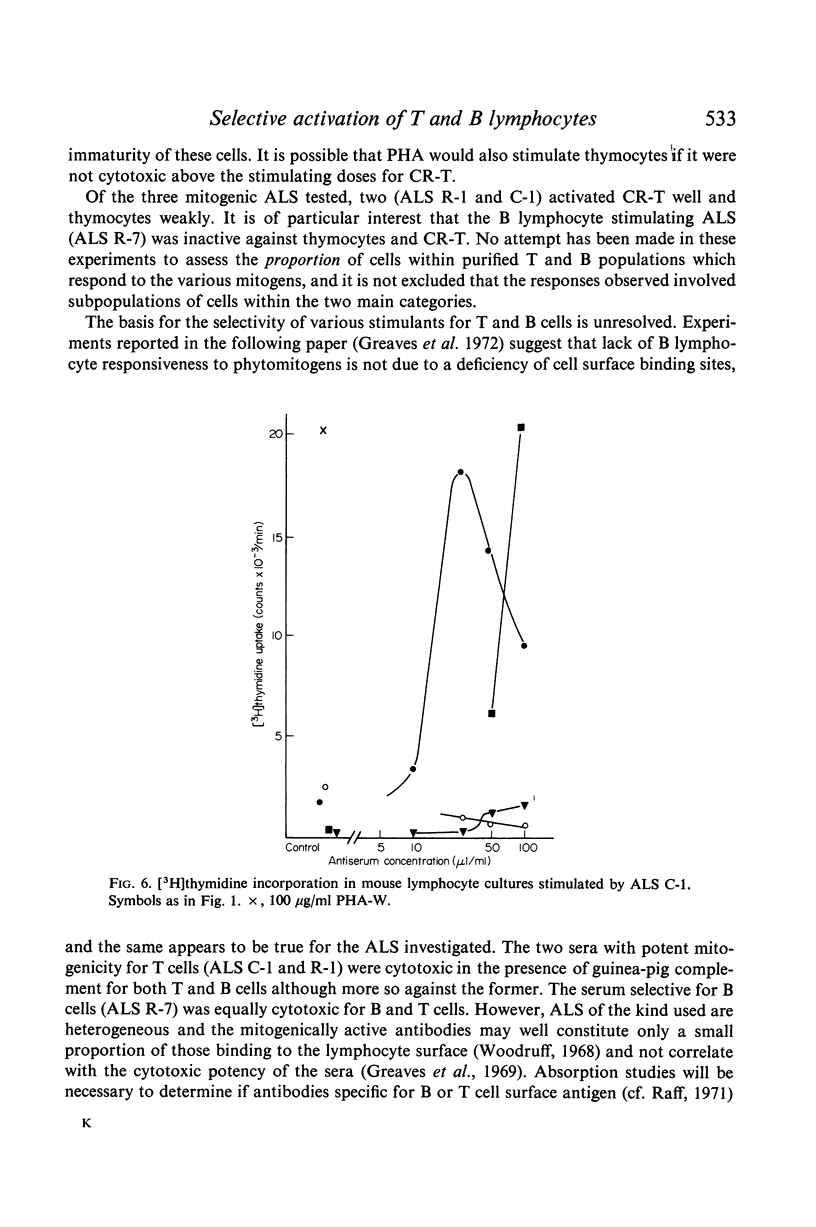
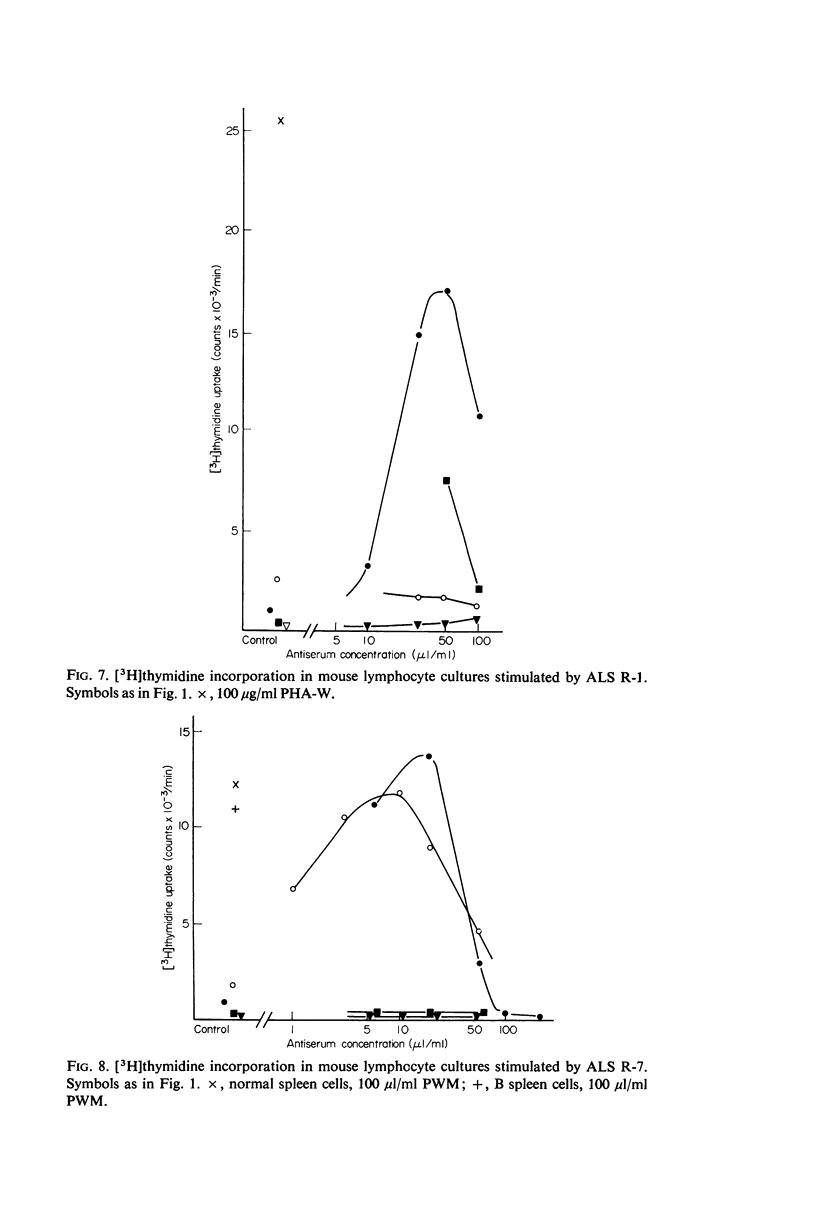
The results show that PHA, ConA and LM stimulate T but not B cells whereas PWM activates both T and B cells. Of six mitogenically active ALS, five were T cell specific and one B cell specific. Cortisone resistant (T) cells within the thymus showed a much greater response to all mitogens than unselected thymocytes. However, ConA and LM activated a considerable response in the latter population.
Selective activation of T and B cells by aspecific means may be useful both as a clinical tool and as an approach to gaining an understanding of antigen induction of immune responses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm G. V., Peterson R. D. Antibody and immunoglobulin production at the cellular level in bursectomized-irradiated chickens. J Exp Med. 1969 Jun 1;129(6):1247–1259. doi: 10.1084/jem.129.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns R. M., Simpson E., Nehlsen S. L., Ruszkiewicz M. A method for producing immunosuppressive antilymphocyte serum in pigs and ruminants. Transplant Proc. 1971 Mar;3(1):784–787. [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Characteristics of the immunocompetent cells in the mouse thymus: cell population changes during cortisone-induced atrophy and subsequent regeneration. Cell Immunol. 1970 Nov;1(5):545–560. doi: 10.1016/0008-8749(70)90041-9. [DOI] [PubMed] [Google Scholar]
- Börjeson J., Reisfeld R., Chessin L. N., Welsh P. D., Douglas S. D. Studies on human peripheral blood lymphocytes in vitro. I. Biological and physicochemical properties of the pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):859–872. doi: 10.1084/jem.124.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenhoff M. J., Davies A. J., Leuchars E., Wallis V. The thymus and circulating lymphocytes of mice. Proc R Soc Lond B Biol Sci. 1970 Oct 13;176(1042):69–85. doi: 10.1098/rspb.1970.0035. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Bauminger S., Janossy G. Lymphocyte activation. 3. Binding sites for phytomitogens on lymphocyte subpopulations. Clin Exp Immunol. 1972 Mar;10(3):537–554. [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F. Biological effects of anti-immunoglobulins: evidence for immunoglobulin receptors on 'T' and 'B' lymphocytes. Transplant Rev. 1970;5:45–75. doi: 10.1111/j.1600-065x.1970.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Tursi A., Playfair J. H., Torrigiani G., Zamir R., Roitt I. M. Immunosuppressive potency and in-vitro activity of antilymphocyte globulin. Lancet. 1969 Jan 11;1(7585):68–72. doi: 10.1016/s0140-6736(69)91089-7. [DOI] [PubMed] [Google Scholar]
- Iványi J., Marvanova H., Skamene E. Immunoglobulin synthesis and lymphocyte transformation by anti-immunoglobulin sera in bursectomized chickens. Immunology. 1969 Sep;17(3):325–331. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Powell A. E., Leon M. A. Reversible interaction of human lymphocytes with the mitogen concanavalin A. Exp Cell Res. 1970 Oct;62(2):315–325. doi: 10.1016/0014-4827(70)90560-4. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Stockman G. D., Gallagher M. T., Heim L. R., South M. A., Trentin J. J. Differential stimulation of mouse lymphoid cells by phytohemagglutinin and pokeweed mitogen. Proc Soc Exp Biol Med. 1971 Mar;136(3):980–982. doi: 10.3181/00379727-136-35410. [DOI] [PubMed] [Google Scholar]
- Woodruff M. F. Purification of antilymphocytic antibody. Nature. 1968 Mar 2;217(5131):821–824. doi: 10.1038/217821a0. [DOI] [PubMed] [Google Scholar]
- Young N. M., Leon M. A., Takahashi T., Howard I. K., Sage H. J. Studies on a phytohemagglutinin from the lentil. 3. Reaction of Lens culinaris hemagglutinin with polysaccharides, glycoproteins, and lymphocytes. J Biol Chem. 1971 Mar 25;246(6):1596–1601. [PubMed] [Google Scholar]