Abstract
The early appearance of serotonin and its receptors during prenatal development, together with the many effects serotonin exerts during CNS morphogenesis, strongly suggest that serotonin influences the development and maturation of the mammalian brain before it becomes a neuromodulator/neurotransmitter. Sites of early serotonin biosynthesis, however, have not been detected in mouse embryos or extraembryonic structures, suggesting that the main source of serotonin could be of maternal origin. This hypothesis was tested by using knockout mice lacking the tph1 gene, which is responsible for the synthesis of peripheral serotonin. Genetic crosses were performed to compare the phenotype of pups born from homozygous and heterozygous mothers. Observations provide the first clear evidence that (i) maternal serotonin is involved in the control of morphogenesis during developmental stages that precede the appearance of serotonergic neurons and (ii) serotonin is critical for normal murine development. Most strikingly, the phenotype of tph1−/− embryos depends more on the maternal genotype than on that of the concepti. Consideration of the maternal genotype may thus help to clarify the influence of other genes in complex diseases, such as mental illness.
Keywords: genotype/phenotype, tph1 knockout mice, tryptophan hydroxylase
Serotonin participates in a wide range of physiological systems including the control of gastrointestinal motility and secretion, cardiovascular regulation, hemostatic processes, the regulation of circadian rhythms, the sleep–wake cycle, perception of pain, appetite, manifestation of nausea, and sexual behavior. Accumulating in vitro evidence also indicates that serotonin signaling participates in the regulation of development in many animal phyla before the onset of neurogenesis. Serotonin thus plays a role in development before it acts as a neurotransmitter (1–3). Serotonin affects craniofacial, gastrointestinal, and cardiovascular morphogenesis in chicken, rat, and mouse; these effects are often mediated by the serotonin 2B receptor (4–7). Altogether, the presence of serotonin, its receptors, and transporter during development and the ability of compounds that modulate serotonergic signaling to interfere with development suggest that serotonin functions as a humoral morphogen (8–10). Sites of early serotonin biosynthesis, however, have not been detected in embryos or extraembryonic structures of the mouse. It has therefore been assumed that the main source of serotonin is maternal (11).
We have generated a mouse line deficient in peripheral serotonin biosynthesis. Targeted disruption of the tryptophan hydroxylase 1 (tph1) gene resulted in levels of circulating serotonin that are only 3–15% of those of normal mice. The null mutants (tph1−/−) from heterozygous crosses are viable and display no gross anatomical abnormalities, but they develop cardiac insufficiency in adulthood (12). The tph1−/− mice thus provide a convenient tool to address the developmental role of maternal serotonin. tph1-null females were bred with tph1 wild-type, heterozygous, or null males. In this study we show that the phenotype of the embryos depends on the genotype of the mothers and not on that of the embryo. Our in vivo work clearly establishes for the first time that maternal serotonin production is an important determinant of normal development. The contribution made by interference with the early availability of maternal serotonin to the pathophysiology of developmental disorders, such as phenylketonuria (PKU) and autism, should now be investigated.
Results
Maternal Serotonin Ensures Normal Development.
To address the requirement for maternally derived serotonin in the developing mouse embryo, wild-type (tph1+/+), heterozygous (tph1+/−), and null mutant (tph1−/−) females were bred with wild-type (tph1+/+), heterozygous (tph1+/−), and null mutant (tph1−/−) males (Table 1).
Table 1.
Phenotype of tph1 embryos depends on maternal genotype
| Maternal genotype | Paternal genotype | No. of crosses | Embryo's genotype |
% of small and/or abnormal embryos | ||
|---|---|---|---|---|---|---|
| +/+ | +/− | −/− | ||||
| +/+ | +/+ | 3 | 28 | — | — | 3.6* |
| +/− | 1 | 4 | 6 | — | 0 | |
| −/− | 2 | — | 27 | — | 3.7* | |
| +/− | +/+ | 1 | 6 | 4 | — | 0 |
| +/− | 4 | 11 | 24 | 10 | 6.7* | |
| −/− | 2 | — | 4 | 3 | 0 | |
| −/− | +/+ | 3 | — | 10 | — | 80† |
| +/− | 1 | — | 9 | 9 | 88.9† | |
| −/− | 2 | — | — | 15 | 86.7† | |
Data show the type (maternal and paternal genotypes) and number of crosses performed to produced the embryos and the number of embryos of wild-type, heterozygous, and homozygous null genotype. All 170 embryos were measured; 104 embryos were further analyzed.
*A total of 3.6–6.7% of embryos obtained from tph1+/+ or tph1+/− females were normal but with smaller CRL (7.4–7.5 mm).
†A total of 80–88.9% of embryos from tph1−/− mothers were smaller (CRL: 5.8–7.4 mm) and presented developmental abnormalities. The remaining 15–20% were normal in size albeit the CRL values were in the lower range. Sagittal cuts presented no gross abnormalities.
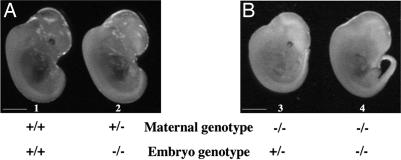
Nineteen different crosses were carried out to obtain each of the possible embryonic genotypes. We first analyzed wild-type, heterozygous, and null mutant embryos from crosses between wild-type and heterozygous parents. Homozygous (tph1−/−) and heterozygous (tph1+/−) embryos obtained from these crosses were indistinguishable from their wild-type littermates (Fig. 1A, embryos 1 and 2). In comparison, the embryos born to tph1−/− mothers, as a result of breeding with a tph1−/−, a tph1+/−, or a wild-type male were significantly decreased in size (Fig. 1B, embryos 3 and 4). Size was evaluated by measuring the crown–rump length (CRL) of the embryos. A 15–30% reduction was observed in embryos obtained from tph1-null mothers as compared with null embryos from heterozygous mothers.
Fig. 1.
Phenotypic comparison of E12.5 embryos from wild-type (A, embryo 1), heterozygous (A, embryo 2), and homozygous (B, embryos 3 and 4) tph1 mothers. Embryos displayed no obvious macroscopic anomalies except for an overall size reduction in embryos obtained from tph1−/− mothers (embryos 3 and 4). Size reduction was evaluated by measuring the CRL of the embryos. An overall 15–30% reduction was observed in the mutant embryos obtained from tph1-null mothers. CRL values are 7.8 mm (embryo 1), 7.7 mm (embryo 2), 6.8 mm (embryo 3), and 7.0 mm (embryo 4). (Scale bars: 0.25 cm.)
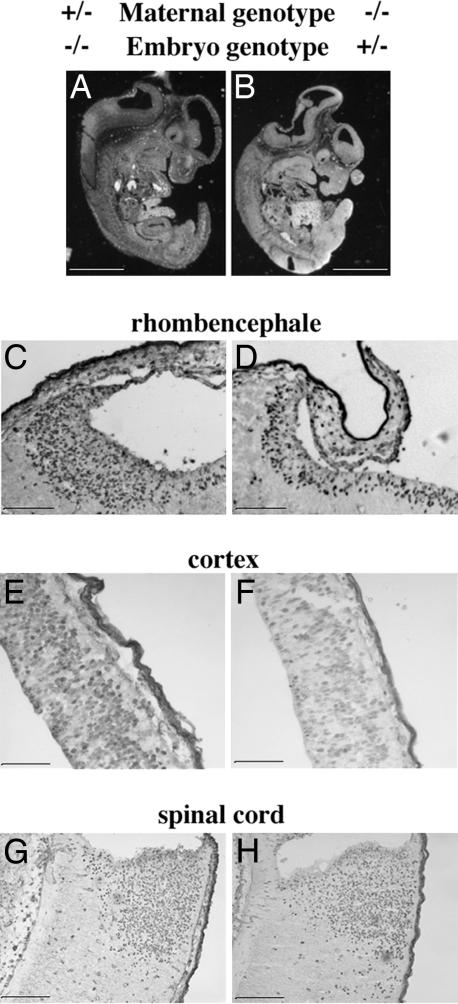
Microscopic investigations were carried out on longitudinal sections of the whole embryos illustrated in Fig. 1. This analysis (Fig. 2A and B) revealed striking abnormalities in the entire brain region. In particular, the shapes of rhombencephale regions and the roof of the neopallial cortex were altered in a heterozygous embryo from a null mother (Fig. 2B). This abnormality was not observed in wild-type, heterozygous, or null embryos obtained from a tph1+/+ or tph1+/− mother bred to a tph1−/−, tph1+/−, or wild-type male (Fig. 2A).
Fig. 2.
Longitudinal section of whole tph1 embryos 2 and 3 of Fig. 1. (A and B) Embryos 2 and 3 were obtained, respectively, from a cross between a tph1+/− mother and a tph1+/− father and a cross between a tph1−/− mother and a wild-type father. Microscopic investigations revealed flattening of the head region at the level of the IV ventricle in the embryos obtained from a null mother. The corresponding CNS area in an embryo obtained from a heterozygous mother showed a normal histology. (C and D) A 2-h BrdU pulse revealed a reduced number (30% less) of labeled cells in the ventricular zone of the heterozygous embryo obtained from a null mother as compared with a null embryo obtained from a heterozygous mother. The analysis also revealed a 24% reduction in BrdU labeling in the roof of neopallial cortex of heterozygous embryos from null mothers (compare F with E). No major difference in BrdU labeling was observed at the level of the spinal cord (G and H). (Scale bars: 0.25 cm in A and B and 100 μm in C–H.)
Most importantly, sagittal sections of heterozygous (tph1+/−) embryos born to a null mother and a wild-type father (Fig. 2B) displayed identical features as a null homozygous embryo (Fig. 1B, embryo 4) obtained from a cross between a tph1−/− female and tph1−/− male (Fig. 2B, and data not shown). The results indicate that the genotype of the mother influences embryonic development regardless of genotype of the embryo. In addition, the observations strongly support the idea that a maternal source of serotonin is necessary for normal development.
To define further the abnormalities observed in the cranial region of embryos obtained from null mothers, a pulse of BrdU was administered 2 h before the embryos were collected. The incorporation of BrdU provided an estimate of mitotic activity. Sites of BrdU incorporation were detected immunocytochemically. These studies revealed that the number of BrdU-immunoreactive cells in the ventricular zone of heterozygous embryos obtained from a tph1-null mother and a wild-type father (Fig. 2D) was 30% less than that of null embryos born to heterozygous parents (Fig. 2C). A 24% reduction in the number of BrdU-positive cells was also observed (Fig. 2 E and F) at the level of the roof of the neopallial cortex (future cerebral cortex). No genotype-related differences were found in the number of BrdU-immunoreactive cells in the spinal cord (Fig. 2 G and H).
Developmental and Tissue-Specific Expression of TPH1 and TPH2.
To test further the hypothesis that maternal rather than embryo-derived serotonin is essential for normal development, we determined the time when the activities of embryonic tph1 and tph2 first gave rise to serotonin. A detailed analysis of transcripts encoding tph1 and tph2 was carried out at different developmental stages by using in situ hybridization (ISH). Specific 3′ UTR probes were used that enabled transcripts encoding tph1 and tph2 to be distinguished. ISH was carried out either in toto or on sagittal sections cut in embryos at embryonic day 8.5 (E8.5) and older. In addition to ISH studies with specific probes in wild-type mice, advantage was taken of the nlsLacZ cassette that was inserted to inactivate the tph1 gene to detect sites of expression of the locus in null mice by demonstrating LacZ expression. Insertion of the nlsLacZ marker into the tph1 locus made it possible to follow the transcriptional activity of the tph1 promoter in cells lacking tph1 expression and to analyze the fate of these cells in the developing mouse (12). Because β-Gal staining faithfully mimicked tph1 expression at each developmental time analyzed, only the TPH1 data are presented (Fig. 3).
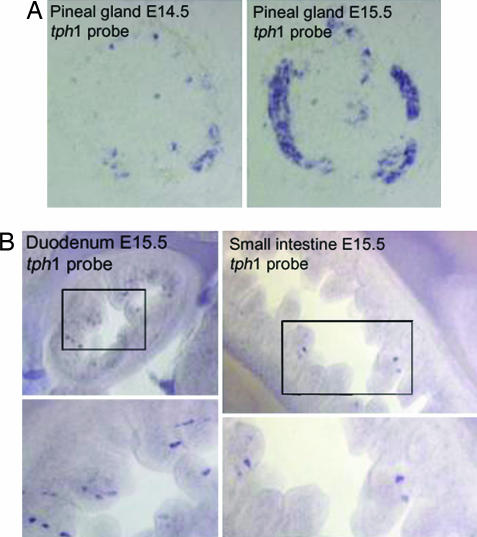
Fig. 3.
mRNA expression pattern of TPH1 in mouse embryos by ISH with a specific 3′ UTR tph1 probe. The expression of the nonneuronal enzyme TPH1 is restricted to the pineal gland and enterochromaffin cells of the gut starting at E14.5 in the pineal gland (A) and at E15.5 in the gut (B).
Strong expression of transcripts encoding tph1 in the pineal gland of wild-type mice was detected beginning at E14.5 (Fig. 3A). In contrast, transcripts encoding tph1 were not detected anywhere in the developing CNS (data not shown). TPH1 expression was observed in the fetal gut for the first time at E15.5 (Fig. 3B). Transcripts encoding tph1 were found in every segment of the gastrointestinal tract below the region of the esophagus. Cells containing transcripts encoding tph1 were present in the epithelium and extended cytoplasmic processes toward the lumen and were thus identified as probable enterochromaffin cells. By immunohistochemical analysis in mutant embryos using antibodies to β-Gal we demonstrated that the numbers of β-Gal-expressing cells increased after birth (unpublished results). This observation is consistent with the known continuous differentiation of enterochromaffin cells from stem cells in crypts throughout life.
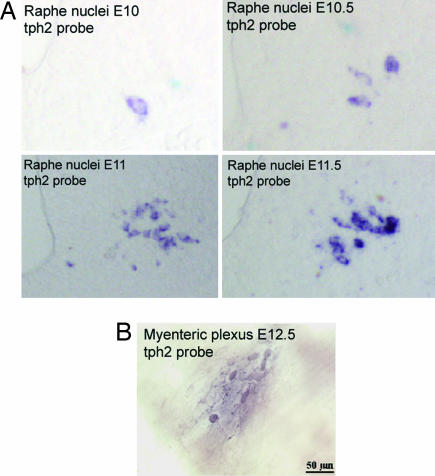
The expression of transcripts encoding TPH2 were investigated in parallel with those encoding TPH1. Transcripts encoding TPH2 were first detected at E10.5 in the developing CNS (Fig. 4A). The first cell in which transcripts encoding TPH2 was observed appeared in the ventral hindbrain in proximity to the floorplate. Transcripts encoding TPH2 were not detected in the pineal gland of either wild-type or tph1−/− embryos during development or thereafter (data not shown). Within the developing bowel (Fig. 4B), as reported previously (13), neurons were recognized as early as E12.5. Subsets of these neurons began to express tph2 at about the same time (E12.5). We have previously demonstrated that tph2 expression is maintained in the neural tissues of adult mice (12). These data provide evidence that the expression of tph2 mRNA is strictly neuron-specific, albeit not brain-specific, even during fetal life.
Fig. 4.
mRNA expression pattern of TPH2 in mouse embryos by ISH with a specific 3′ UTR tph2 probe. The neuronal enzyme TPH2 is expressed in the developing nervous system at early embryonic stages (E10.5) (A) and in neurons of the myenteric plexus at E12.5 (B).
The detailed analysis of tph1 and tph2 expression during fetal life clearly pinpoints a tissue-specific developmental expression pattern of the two TPH isoforms. Previous studies have indicated that in the mouse central serotonergic neurons can first be detected at E11.5 with anti-serotonin antibodies (14–16). Our results further show that the serotonin detected at this stage is likely to be synthesized by the newly expressed TPH2. TPH2-dependent embryo-derived serotonin at E11.5, however, is not sufficient to prevent abnormal phenotypic expression in tph1−/− embryos born to a tph1−/− mother. The TPH1-derived maternal source of serotonin is therefore an important contributor to the normal appearance of tph1-null embryos.
Discussion
Phenotype of tph1-Null Mutant Mice Depends on the Mother's Genotype.
In humans, as well as in most other mammalian species, serotonin is produced by two distinct enzymes, TPH1 and TPH2. TPH1, which is located in the pineal gland and gut enterochromaffin cells, is responsible for synthesizing most of the serotonin found in the body (17, 18). TPH2, which is restricted to neurons of the raphe nuclei and the enteric nervous system, is responsible for the synthesis of the remaining serotonin. Approximately 95% of the body's serotonin is found in the bowel; >90% of that is produced by enterochromaffin cells and thus is synthesized by TPH1. Virtually all of the serotonin in the bloodstream is located in blood platelets, which do not themselves synthesize serotonin. Platelets take up overflow serotonin from the gut and contain no serotonin in knockout mice lacking the plasmalemmal serotonin transporter (SERT) (19). TPH1, therefore, is indirectly responsible for synthesis of blood serotonin. Recently, tph1−/− homozygous mice have been generated from heterozygous parents. These animals are viable and show no gross anatomical alterations. As adults, however, tph1−/− mice develop cardiac insufficiency, which in some conditions may lead to death (12). Gastrointestinal motility is also slowed in tph1−/− mice.¶
In the current work genetic crosses were carried out to investigate pups born to tph1-null mothers. Quite remarkably, these pups displayed dramatic abnormalities in the development of the brain and other tissues, irrespective of whether they were tph1+/− or tph1−/−; therefore, the phenotype of the pups must not be linked to their own tph1 genotype but to that of their mothers. These findings, confirmed by histological investigations, provide the first unambiguous demonstration that physiologically significant serotonin of maternal origin must be present in embryos before E11. This maternally derived serotonin is essential for the normal development of fetuses. Serotonin found in blood platelets was previously shown to be reduced in tph1−/− as compared with tph1+/− females (12). Values for circulating serotonin in tph1+/− females were the same as in wild-type females but dropped to 3–15% of wild-type in tph1−/− females (12). Embryos of tph1-null mothers are exposed to very low levels of serotonin during early embryogenesis, irrespective of the tph1 genotype of the embryos. Affected animals display major alterations in their development. In sharp contrast, pups that are born to heterozygous mothers have been exposed to a normal (maternal-derived) blood level of serotonin. These animals appear to be normal at birth, no matter whether their genotype is tph1−/− or tph1+/−.
The importance of the maternal genotype suggests that there is a threshold serotonin level that must be achieved in order for embryos to develop normally. As shown in Table 1, however, 15–20% of null mice from null mothers were reduced in size but displayed no gross abnormalities (at least at this specific developmental time). Although smaller embryos (6.7%) were obtained from crosses between wild-type or heterozygous mothers, the gross abnormalities characteristic of embryos derived from tph1-null mothers were not observed. As presented in Table 1, 27 tph1+/−embryos were obtained and studied from three different crosses between three tph1−/− males and three tph1+/+ females. As a result, one embryo was smaller (CRL: 7.4 mm), but again sagittal sections revealed none of the gross abnormalities observed in the null embryos obtained from null mothers. In parallel, seven embryos were obtained and studied from one cross between a tph1−/− male and a tph1+/− female. The three tph1−/− and four tph1+/− embryos obtained from this cross appear normal at both the macroscopic and microscopic levels. All together, these results argue for a maternal effect on fetal development and against a major effect of a paternal imprinting contribution. An influence on embryonic development of putative additional factors such as the environment cannot, however, be excluded.
The current analysis of the expression of tph1 and tph2 established that the serotonin pool found very early in embryos is unlikely to be produced endogenously. Transcripts encoding TPH2 could not be detected until E10.5, a time that precedes the appearance of TPH2 protein by a half day. No transcripts encoding TPH2 were detected in the pineal gland of embryos. Transcripts encoding TPH2 were also detected starting at E12.5 within developing enteric neurons. Sites of tph1 expression were demonstrated by ISH as well as by taking advantage of the expression of the nlsLacZ marker inserted in place of the tph1 gene in tph1-null mice. Expression of transcripts encoding TPH1 appears in the pineal gland at E14.5 and in the fetal gut at E15.5 at all levels below the esophagus. Finally, transcripts encoding TPH1 could not be detected in the brain at any stage of development. Clearly, the endogenous production of serotonin in the developing embryo occurs much later than that of other serotonergic signaling molecules. For example, the serotonin 2B receptor and the plasmalemmal SERT have been detected as early as E8–9 (5–8, 11). Furthermore, recent data in both chick and frog embryos have suggested that serotonin 2B receptors as well as the SERT and the serotonin-degrading enzyme MAO are involved in the early steps that pattern left–right axis (3). This indicates that serotonin is likely to be available in embryos long before the expression of Shh in the floor plate and thus before serotonergic neurons develop (3, 8).
Maternal Serotonin May Be a Morphogen-Like Signaling Molecule.
Serotonin has been thought to act as a morphogen in early stages of development. For example, studies using cultured embryos have implicated serotonin in neural crest migration, craniofacial and limb development, mesenchymal cell proliferation, and the specification of neuronal identity and connectivity. The use of uptake inhibitors in cultured embryos also supports this view (ref. 3 and references therein and ref. 21). Serotonin may thus participate in the shaping and/or maintenance of particular brain structures during early fetal life. These postulated roles of serotonin have now been investigated by using genetic tools. The reduced number of BrdU-labeled cells around the ventricular zone in embryos obtained from tph1-null mothers indicates that there is a lower-than-normal rate of mitosis in the neuroepithelium and provides evidence that abnormalities in serotonin signaling during development can perturb craniofacial development. Still unclear is the duration of embryonic life during which maternal serotonin is available to embryos and participates in their development. Intrinsic embryonic serotonin, which starts to be produced at E11 when tph2 expression begins, could play a morphogenetic role in subsequent fetal life. In any case, it should be stressed that during development the tph2 gene of the embryo is likely to act in concert with the tph1 gene of the mother; moreover, the tph1 gene from the fetus, which is expressed in the pineal gland and in the gut, could also participate in brain development after its expression begins. The facts that the blood–brain barrier is incomplete during early fetal life and that mechanisms to prevent the circulation of free serotonin are immature probably allow serotonin transferred from the mother to the fetal circulation to be physiologically active and influence development. How maternal serotonin is transferred to fetuses is unclear. Almost all circulating serotonin is present in platelets, and free blood serotonin is very low even when platelets lack the SERT and cannot take up serotonin (19). There may thus be a mechanism that permits platelet serotonin to be released for transplacental transfer to the fetus.
The concept of a maternal effect on fetal development is not without precedent. In 1994, Letterio et al. (22) showed that maternal sources of TGFβ1 contribute to the normal appearance and perinatal survival of TGFβ-null newborn mice (22). Immunohistochemical analyses revealed that TGFβ-null embryos and null newborn pups born to TGFβ heterozygotes were TGFβ-immunoreactive, whereas those born to a null female were not TGFβ-immunoreactive and also displayed severe cardiac abnormalities. In contrast to TPH1, however, TGFβ heterozygous embryos born to null mothers showed no gross abnormalities. The maternal effect of TGFβ serves to rescue fetuses from targeted intrinsic gene disruption. In comparison, maternal serotonin evidently exerts a physiological effect on ontogeny because its availability is required for normal fetal development. The genetic makeup of the mother supersedes that of the concepti. Such a situation is likely to occur in other instances, and their consideration may help clarify the influence of particular genes in complex diseases.
Serotonergic Abnormalities During Development.
The observation that serotonin of maternal origin plays a critical role in embryonic development may help to rationalize disparate clinical observations and have therapeutic implications. PKU is an example. The maternal contribution of serotonin may explain the occurrence of some of the defects observed in babies born to untreated mothers with PKU. PKU is a recessive inborn error of metabolism caused by a deficiency of the hepatic enzyme phenylalanine hydroxylase. Phenylalanine, tyrosine, and tryptophan hydroxylases constitute a closely related family of enzymes. Dietary treatment during childhood is designed to lower the phenylalanine level to prevent neurological damage. If treatment is not resumed during pregnancy, brain and cardiac defects will occur in fetuses (23, 24). An excess of phenylalanine leads to a secondary inhibition of both TPH and TH, thereby resulting in a severe decrease of serotonin in blood and cerebrospinal fluid in mothers with PKU and also in their fetuses. It is thus possible that it is the decrease in the serotonin's availability to the developing embryo that accounts for the brain and cardiac abnormalities observed in the children of mothers with untreated PKU.
Autism is another example in which the maintenance of a proper level of serotonin may be critical for normal brain development. The pathophysiology of autism is unknown, but autism is thought to be a “disease of development” (25). Serotonin may be involved in the pathophysiology of autism because autism is frequently, although not invariably, associated with an elevated blood serotonin level. This inconsistency may reflect the fact that autism is diagnosed as a symptom complex and thus may represent a heterogeneous category of disease; nevertheless, because all of the serotonin found in blood platelets is derived from the bowel, the observation suggests that there is a defect, at least in affected individuals, in the gut as well as in the brain. The SERT, which mediates serotonin uptake by blood platelets as well as enteric and central serotonergic neurons, has also been implicated in autism (26, 27), although recent studies have not shown a correlation between SERT polymorphisms and autism (28, 29). Janusonis (30) has developed a mathematical model that involves a factor interfering with brain development in autism and which also regulates serotonin release or synthesis. This theory has not been proven. It is conceivable that variations in maternal serotonin levels exert subtle effects on brain development during early ontogeny irrespective of the proper levels of peripheral serotonin in the affected child. Abnormal serotonin levels have been detected in the blood of parents of autistic children even when these parents are not themselves autistic (31). These considerations, however, do not exclude the possibility that an imbalance in the central or peripheral serotonin levels in a child could also contribute to the autistic phenotype. Autism thus might be a disease for which genetic analyses would benefit from taking into account the maternal tph1 genotype and blood serotonin levels during pregnancy of the mother in addition to the tph1 and tph2 genotypes of the offspring.
Finally, serotonin plays an important role in the regulation of gastrointestinal activity, which is not surprising because the bowel contains by far the body's largest pool of serotonin (18). Serotonin and SERT also appear to play roles in the pathophysiology of functional disorders of gastrointestinal motility, including irritable bowel syndrome (IBS) (18, 32). IBS affects as many as 20% of the population. The cause of IBS is unknown, but it has been demonstrated to be associated with decreased SERT expression in the gastrointestinal mucosal epithelium (33). SERT develops before fetal expression of TPH1 or TPH2 is evident, and thus, for mucosal SERT expression to be influenced by serotonin, the source of the serotonin would have to be maternal. IBS does appear to have a familial contribution, although twin studies have shown only a modest contribution of genetics (34). It is thus possible that maternal serotonin can contribute to fetal bowel development and account for a familial transmission that is independent of the genetic makeup of the fetus.
Materials and Methods
Mouse Embryos.
All animal experiments were conducted in accordance with the French Ministère de l'Agriculture et de la Forêt and the European Community guidelines for the care and use of animals. The tph1 gene was disrupted in embryonic stem cells as previously described (12). The tph1-null mutation was backcrossed onto a C57BL/6 background. Embryos were obtained from crosses between TPH1 wild-type, heterozygous, and homozygous males bred to TPH1 wild-type, heterozygous, and homozygous females. The day at which the vaginal mating plug was seen was taken as E0.5. For BrdU incorporation, pregnant females were injected intraperitoneally (2 g/kg) 2 h before sacrifice. Embryos were fixed with 4% paraformaldehyde in PBS 1× buffer overnight. After fixation, embryos were rinsed twice in PBS 1× buffer and were subsequently cryoprotected in 15% sucrose overnight and frozen. Sagittal sections covering the entire embryo were cut at 16 μm with a cryotome. BrdU incorporation was measured by using ImageJ software.
In Situ Hybridization and Immunohistochemistry.
ISH using specific 3′ UTR sequences of tph1 and tph2 was used for riboprobe synthesis as described by Côté et al. (12). Immunohistochemistry using rat anti-BrdU was carried out as described by Ravassard et al. (20).
Acknowledgments
We thank Hélène Kiefer, Jeannette Nardelli, and Martin Catala for helpful discussions and Jack Formentin for technical assistance. C.F. was supported by a fellowship from the French Research Ministry. This work was supported by the Centre National de la Recherche Scientifique, Université Pierre et Marie Curie, Sanofi-Aventis, the Association Française Contre les Myopathies, the Conseil Régional d'Ile de France, the Institut pour la Recherche sur la Moëlle Epinière, and INSERM.
Abbreviations
- CRL
crown–rump length
- TPH
tryptophan hydroxylase
- SERT
serotonin transporter
- ISH
in situ hybridization
- PKU
phenylketonuria
- En
embryonic day n.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
¶Chen, J. J., Wan, S., Côté, F., Mallet, J., Gershon, M. D. (2005) Auton Neurosci 119:78 (Abstr.).
References
- 1.Moiseiwitsch JR. Crit Rev Oral Biol Med. 2000;11:230–239. doi: 10.1177/10454411000110020601. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- 3.Levin M, Buznikov GA, Lauder JM. Dev Neurosci. 2006;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- 4.Wallace JA. J Comp Neurol. 1985;236:443–453. doi: 10.1002/cne.902360403. [DOI] [PubMed] [Google Scholar]
- 5.Choi DS, Ward SJ, Messaddeq N, Launay J-M, Maroteaux L. Development (Cambridge, UK) 1997;124:1745–1755. doi: 10.1242/dev.124.9.1745. [DOI] [PubMed] [Google Scholar]
- 6.Fiorica-Howells E, Maroteaux L, Gershon MD. J Neurosci. 2000;20:294–305. doi: 10.1523/JNEUROSCI.20-01-00294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buznikov GA, Lambert HW, Lauder JM. Cell Tissue Res. 2001;305:177–186. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- 8.Gaspar P, Cases O, Maroteaux L. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 9.Lauder JM. Prog Brain Res. 1988;73:365–387. doi: 10.1016/S0079-6123(08)60516-6. [DOI] [PubMed] [Google Scholar]
- 10.Lauder JM, Zimmermann EF. J Craniofac Genet Dev Biol. 1988;8:265–276. [PubMed] [Google Scholar]
- 11.Yavarone MS, Shuey DL, Tamir H, Sadler TW, Lauder JM. Teratology. 1993;47:573–584. doi: 10.1002/tera.1420470609. [DOI] [PubMed] [Google Scholar]
- 12.Côté F, Thévenot E, Fligny C, Fromes Y, Darmon M, Ripoche M-A, Bayard E, Hanoun N, Saurini F, Lechat P, et al. Proc Natl Acad Sci USA. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothman TP, Gershon MD. J Neurosci. 1982;2:381–393. doi: 10.1523/JNEUROSCI.02-03-00381.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goridis C, Rohrer H. Nat Rev Neurosci. 2002;7:531–541. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- 15.Wallace JA, Lauder JM. Brain Res Bull. 1983;4:459–479. doi: 10.1016/0361-9230(83)90144-2. [DOI] [PubMed] [Google Scholar]
- 16.Cheng L, Chen CL, Luo P, Tan M, Qiu M, Johnson R, Ma Q. J Neurosci. 2003;23:9961–9967. doi: 10.1523/JNEUROSCI.23-31-09961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erspamer V. In: Handbook of Experimental Pharmacology. Erspamer V, editor. New York: Springer; 1966. pp. 132–181. [Google Scholar]
- 18.Gershon MD. J Clin Gastroenterol. 2005;39:S184–S193. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- 19.Chen JJ, Zhishan L, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravassard P, Chatail F, Mallet J, Icard-Liepkalns C. J Neurosci Res. 1997;48:146–158. doi: 10.1002/(sici)1097-4547(19970415)48:2<146::aid-jnr7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 21.Nebigil CG, Choi D-S, Dierich A, Hickel P, Le Meur M, Messaddeq N, Launay J-M, Maroteaux L. Proc Natl Acad Sci USA. 2000;97:9509–9513. doi: 10.1073/pnas.97.17.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Science. 1994;264:1936–1938. doi: 10.1126/science.8009224. [DOI] [PubMed] [Google Scholar]
- 23.Roux C, Madani M, Launay J-M, Rey F, Citadelle D, Mulliez N, Kolf M. Toxicol In Vitro. 1995;9:653–662. doi: 10.1016/0887-2333(95)00071-f. [DOI] [PubMed] [Google Scholar]
- 24.Ormazabal A, Vilaseca MA, Perez-Duenas B, Lambruschini N, Gomez L, Campistol J, Artuch R. J Inherited Metab Dis. 2005;28:863–870. doi: 10.1007/s10545-005-0153-3. [DOI] [PubMed] [Google Scholar]
- 25.Scott MM, Deneris ES. Int J Dev Neurosci. 2005;23:277–285. doi: 10.1016/j.ijdevneu.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 26.McCauley JL, Olson LM, Dowd M, Amin T, Steele A, Blakely RD, Folstein SE, Haines JL, Sutcliffe JS. Am J Med Genet B. 2004;127:104–112. doi: 10.1002/ajmg.b.20151. [DOI] [PubMed] [Google Scholar]
- 27.Coutinho AM, Oliveira G, Morgadinho T, Fesel C, Macedo TR, Bento C, Marques C, Ataide A, Miguel T, Borges L, Vicente AM. Mol Psychiatry. 2004;9:264–271. doi: 10.1038/sj.mp.4001409. [DOI] [PubMed] [Google Scholar]
- 28.Persico AM, Pascucci T, Puglisi-Allegra S, Militerni R, Bravaccio C, Schneider C, Melmed R, Trillo S, Montecchi F, Palermo M, et al. Mol Psychiatry. 2002;7:795–800. doi: 10.1038/sj.mp.4001069. [DOI] [PubMed] [Google Scholar]
- 29.Wu S, Guo Y, Jia M, Ruan Y, Shuang M, Liu J, Gong X, Zhang Y, Yang J, Yang X, Zhang D. Neurosci Lett. 2005;381:1–5. doi: 10.1016/j.neulet.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 30.Janusonis S. Theor Biol Med Model. 2005;19:2–27. doi: 10.1186/1742-4682-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leboyer M, Philippe A, Bouvard M, Guilloud-Bataille M, Bondoux D, Tabuteau F, Feingold J, Mouren-Simeoni MC, Launay J-M. Biol Psychiatry. 1999;45:158–163. doi: 10.1016/s0006-3223(97)00532-5. [DOI] [PubMed] [Google Scholar]
- 32.Mawe GM, Coates MD, Moses PL. Aliment Pharmacol Ther. 2006;23:1067–1076. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- 33.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Saito YA, Petersen GM, Locke GR, Talley NJ. Clin Gastroenterol Hepatol. 2005;3:1057–1065. doi: 10.1016/s1542-3565(05)00184-9. [DOI] [PubMed] [Google Scholar]