Abstract
Because the flowering and fruiting phenology of plants is sensitive to environmental cues such as temperature and moisture, climate change is likely to alter community-level patterns of reproductive phenology. Here we report a previously unreported phenomenon: experimental warming advanced flowering and fruiting phenology for species that began to flower before the peak of summer heat but delayed reproduction in species that started flowering after the peak temperature in a tallgrass prairie in North America. The warming-induced divergence of flowering and fruiting toward the two ends of the growing season resulted in a gap in the staggered progression of flowering and fruiting in the community during the middle of the season. A double precipitation treatment did not significantly affect flowering and fruiting phenology. Variation among species in the direction and magnitude of their response to warming caused compression and expansion of the reproductive periods of different species, changed the amount of overlap between the reproductive phases, and created possibilities for an altered selective environment to reshape communities in a future warmed world.
Keywords: climate change, global warming, precipitation
Phenology is a sensitive biosphere indicator of climate change (1, 2). Long-term surface data and remote sensing measurements indicate that plant phenology has been advanced by 2–3 days in spring and delayed by 0.3–1.6 days in autumn per decade (3–6) in the past 30–80 years, resulting in extension of the growing season. An extended growing season leads to increased production in terrestrial and marine ecosystems (7, 8), widens amplitudes of the annual CO2 cycle in the atmosphere (9), and prolongs production of allergic pollens (10). Although changes in vegetative phenology have considerable consequences for ecosystem functioning, we lack information on responses of reproductive phenology due to climate change, especially in a community setting (11, 12). Reproductive events usually determine population and community dynamics in future generations, affecting evolutionary processes. Because the flowering and fruiting phenology of plants is very sensitive to environmental cues such as temperature, moisture, and photoperiod (13), it is imperative to understand the impact of climate change on reproductive phenology.
Reproductive phenology of assembled species in a plant community is often staggered in an unbroken progression over the growing season (14–17). This temporal distribution of community-level reproductive events is largely generated by the different developmental trajectories and life forms of the different species and may be shaped by their resource needs during reproduction and ecological sorting (18). Phenological differences in reproductive events among species over the growing season may reduce competition by spreading primary resource use over different temporal pools (19–21). Differential changes in phenology and growth between species in response to climate change could lead to new patterns of species coexistence during reproduction, potentially affecting competitive interactions and, ultimately, the species composition of the community (22–24).
To examine the phenological responses of prairie plants to warming and extra precipitation, we monitored the flowering and fruiting phenology of 12 species over an entire season from March to November 2003 in a tallgrass prairie in the south central Great Plains in the United States. We observed five winter annual species [four forbs (Viola bicolor, Veronica arvensis, Cerastium glomeratum, and Plantago virginica) and one C3 grass (Bromus japonicus)]; one biennial forb (Erigeron strigosus); and six perennials [two forbs (Achillea millefolium and Ambrosia psilostachya), one C3 grass (Dichanthelium oligosanthes), and three C4 grasses (Panicum virgatum, Andropogon gerardii, and Schizachyrium scoparium)]. These species were part of an experiment with four treatments: (i) control with ambient temperature and ambient precipitation, (ii) doubled precipitation with ambient temperature, (iii) warming with ambient precipitation, and (iv) warming plus doubled precipitation.
Results and Discussion
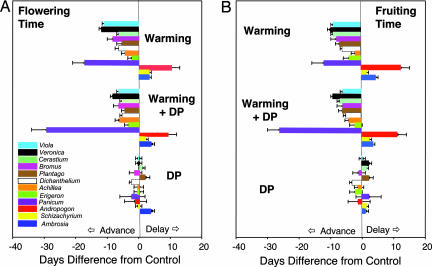
Our experiment showed that warming resulted in substantial divergence in plant reproductive phenology between species on either side of the summer temperature peak (Figs. 1 and 2). The nine species that started flowering before peak summer temperature (hereafter referred to as early-blooming species) advanced their reproductive phenology, whereas the three species that started flowering after peak summer temperature (hereafter referred to as late-blooming species) delayed their reproductive phenology under warming in comparison to the control [Figs. 1 and 2 and supporting information (SI) Table 1]. The mean advance in flowering and fruiting times averaged over the nine early-blooming species was 7.6 days (P < 0.05). The phenological advance was statistically significant for all of the individual species except E. strigosus (SI Tables 1 and 2). This latter species also did not show changes in flowering phenology in an observational study over a time span of 60 years, during which global mean temperatures increased by 0.6°C (25). In contrast, flowering and fruiting times in the three late-blooming species were delayed by an average of 4.7 days (P < 0.05). Despite small differences, the warming-caused delay was statistically significant for flowering of S. scoparium (2.5 ± 1 days, P < 0.05) and fruiting of A. psilostachya (3 ± 1 days, P < 0.05) because of low variation (Figs. 1 and 2 and SI Tables 1 and 2).
Fig. 1.
Changes in the onset of flowering (A) and fruiting (B) (in days) in three experimental treatments [i.e., warming, doubled precipitation (DP), and warming plus DP] compared with the control. Species are listed in the order buds were first observed in control plots, beginning in March with V. bicolor and ending in late August with A. psilostachya. A positive value indicates earlier flowering or fruiting than the control; a negative value indicates later flowering or fruiting than the control. For the warming and warming plus DP treatments, the differences in flowering and fruiting from the control are significant for all species except Erigeron (P < 0.05). In the DP treatment, there were no significant differences in the onset of flowering and fruiting from the control. Data are mean ± SE for advanced or delayed phenology, respectively.
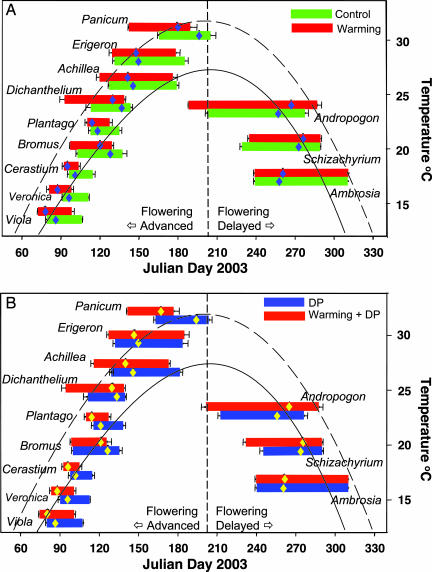
Fig. 2.
Timing and duration of the entire reproductive period composed of three phases (budding, flowering, and fruiting) for the 12 species under four treatments. The diamond symbol indicates the averaged starting date of flowering, from which differences of flowering times between warming and control treatments were calculated as presented in Fig. 1. Fruiting times were similarly analyzed. Lengths of the three phases are presented in SI Table 3. In A, green indicates control, and red indicates warming. In B, blue indicates doubled precipitation (DP), and red indicates warming plus DP. In A, the solid curve is the fitted polynomial regression to daily temperature in the control plots and the dashed curve is for the warmed plots. In B, the solid curve is for the DP, and the dashed curve is for the warming plus DP. The dotted vertical line indicates the peak summer temperature as defined by the maximum temperature of the regression curve. For Cerastium, Dichanthelium, Panicum, and Andropogon, changes in timing or duration caused by the warming and DP treatments are large enough to significantly affect the overlap between paired species (see text). Data are presented as duration ± SE at the two ends of reproductive periods.
Contrary to reports that the earliest flowering species have the largest phenological response to warming (12, 13, 25), we observed the greatest response from the two species flowering in mid-summer. Panicum advanced its flowering time by 17 days (P < 0.05) and its fruiting time by 12 days (P < 0.05), and Andropogon delayed flowering 10 days (P < 0.05) and fruiting 12 days (P < 0.05) in the warmed plots compared with controls (Fig. 1A). By advancing flowering and fruiting in the early-blooming species and delaying them in the late-blooming species, experimental warming drove apart the reproductive events of different species, away from the middle of the season in the tallgrass prairie community.
The combined warming and doubled precipitation treatment resulted in changes in reproductive phenology that were similar to those under warming alone for all species except Panicum. The latter started flowering an average of 29 days (P < 0.05) earlier in the combined treatment than in the control (Fig. 1 and SI Table 1). Doubled precipitation did not significantly affect flowering or fruiting phenology in our grassland ecosystem as it has for trees of seasonal tropical forests and some desert plants (13, 16).
Both observational and experimental studies of flowering phenology usually focus on spring and early-summer flowering plants, which typically show advanced flowering in warmer years (2–6, 12, 25–30). A few studies have found extensions of flowering into fall for late-blooming species (31) and delayed fruiting in warmer years (27, 30, 32), although advanced fruit ripening is more common (27, 30). This study revealed a clear pattern of flowering advance for all species blooming before peak summer heat and delay for all species blooming after the peak of summer heat. The delay we observed in this study corresponds to the commonly reported delay of leaf senescence in autumn in warmer years (1, 3).
For the nine early blooming species, the advance of flowering and fruiting under warming was also reflected by the earlier appearance of buds, due to earlier emergence of perennials and increased growth in warmed plots compared with controls. For late-blooming species, however, the delay of the reproductive phenology was not related to the time of bud appearance. The three late-blooming perennials emerged earlier and were in the bud stage at the same time (Ambrosia and Schizachyrium) or earlier (Andropogon) than those in unwarmed plots but flowered and fruited later in warmed plots (Figs. 1 and 2). Thus, the delay of flowering and fruiting in the late-blooming species was largely due to a prolonged bud stage (e.g., prolonged by 21 days, P < 0.05 for Andropogon; SI Table 3).
The phenological divergence we observed potentially results from a variety of mechanisms. One is a differential response of plant development to warming at different ambient temperatures. Warming in spring may have increased developmental rates of early-blooming species. In summer, when temperature is already very high, warming may have exceeded optimal ranges for development of reproductive tissues and slowed or completely suspended development in the late-blooming species. As a consequence, warming resulted in a divergence in reproductive phenology between grassland species. Other possible mechanisms are size-dependent floral induction, growth responses to warming (instead of or in addition to developmental responses), or soil drying. In our experiment, soil moisture content was similar in the warming plus doubled precipitation plots and the control plots during the late summer and fall, yet phenology was still affected (Fig. 1). Thus, warming, rather than soil drying, was probably the major phenological cue in our study. Plant biomass did respond to both warming and precipitation treatments in our experiment, but doubled precipitation did not induce significant changes in phenology. Thus, growth and size may not be the primary triggers inducing phonological divergence by warming.
The observed phenological divergence in this grassland under warming was accompanied by the formation of a phenological gap during the summer, compression or expansion of temporal reproductive periods, and alteration of the amount of overlap in the reproductive phase between successive species pairs. The reproductive periods of the 12 species surveyed were staggered, forming a progression over the entire growing season from March to November (Fig. 2). The flowering date for Panicum, the species blooming just before the peak of summer, was 61 days earlier than that of Andropogon, the species flowering after the peak of summer, in the control. Experimental warming drove the flowering and the fruiting dates of the two summer species farther apart, by 27 and 24 days (P < 0.05), respectively (Figs. 1 and 2). Although warming did not significantly affect reproductive overlap between the two summer species, their reproductive phases were separated by a gap of 9.1 ± 3.2 days (P < 0.05) with doubled precipitation and 24.7 ± 5.6 days (P < 0.05) in the warming plus doubled precipitation treatment. This formation of a reproductive gap could possibly create a new niche in mid-summer for heat-tolerant species, which may be conducive to invasion by nonnative species.
Experimental warming also significantly changed the duration of the reproductive phases of 7 of the 12 species, leading to compression or expansion of their temporal reproductive periods (Fig. 2 and SI Tables 1 and 3). Perennials in this experiment generally have a longer reproductive duration than annuals. The reproductive durations of Veronica, Cerastium, Plantago, and Schizachyrium were significantly shortened by 3, 6.5, 5, and 5 days (P < 0.05), respectively, under warming in comparison to controls. The 6.5-day reduction in the duration of Cerastium reproduction with warming represents a shortening of the reproductive period by 36% compared with the control. This compression may exert selective pressure for genetic changes and adaptive evolution. The success in evolutionary adaptation of these species to climate change, however, will hinge largely upon whether genetic correlations among traits are antagonistic or reinforcing (33). In contrast, the reproductive durations of Dichanthelium, Panicum, and Andropogon were lengthened by 16, 7, and 24 days (P < 0.05), respectively, under warming in comparison to those in the control. The expanded reproductive periods may be evolutionarily beneficial to some species with longer development time but could also make those species more vulnerable to drought and other stresses with future warming. Warming plus double precipitation caused similar compression and expansion of reproductive periods, although double precipitation alone did not (Fig. 2 and SI Tables 1 and 3).
Warming significantly affected the temporal overlap of reproductive stages between successively blooming species, which could alter their competitive relationships during reproduction (34, 35) (Fig. 2 and SI Table 4). The advance in flowering of Dichanthelium caused its reproductive period to overlap with Viola and Veronica by 8 days each (P < 0.05) and resulted in 14 days greater overlap with Bromus (P < 0.05) in warmed plots than in unwarmed plots (Fig. 2 and SI Table 4). Warming advanced the reproductive phenology of Veronica more than that of Bromus and hence decreased the reproductive overlap between the two species by 7 days (P < 0.05). Doubled precipitation affected the reproductive overlap of six species pairs, whereas the warming plus doubled precipitation treatment significantly changed overlap of five species pairs (Fig. 2 and SI Table 4). Although most of the changes in overlap between the species pairs we studied in this grassland do not involve competition for pollinators (except possibly Veronica and Cerastium), changes in reproductive overlap can impact relative fitness of species through competition for other resources such as water, nutrients, and light (36). During the reproductive period of bud growth, flowering, and fruiting, plant demand for resources is usually high (34, 35, 37). Some species even increase their nutrient uptake during this period (38, 39). Modifications in the timing of flowering are known to affect flower size, number, and seed set (40–42), which in turn affect reproductive fitness (43).
Although the experimental design for a 1-year pulse warming experiment limited our observation to one growing season, the seasonal temperature pattern and annual mean in 2003 were similar to the 30-year average at our site. Mean annual precipitation was below the 30-year average, being relatively dry in April, July, October, and November. The dry year should have accentuated the effects of the double precipitation treatment. Instead we found no effect of added precipitation on phenology. Observations of climate and plant phenology in Europe have not found any noticeable correlation between rainfall and phenology (26, 27), and another grassland field experiment showed no significant phenological response to precipitation (44). Low precipitation and soil moisture in 2003 may not have strong effects on the phenological patterns we observed. Moreover, the advance of flowering and fruiting phenology in spring under warming is supported by numerous studies (2–6, 12, 25–30), and delayed fruit ripening has also been observed (27, 30). Thus, our observed divergence of reproductive phenology was unlikely to have resulted from peculiar climate events in 2003 but has yet to be examined at other sites and over years.
A community pattern of blooming in which the flowering of various species is staggered in an unbroken progression over a growing season has been widely observed in forests, grasslands, and arid lands (14–17). The pattern may indicate a dynamic evolutionary consequence of the reproductive phenology of the species and the resources available in the environments to which they have been adapted (18). Such a consequence is usually achieved via multiple evolutionary processes, such as character displacement, mutualistic partnering, phenotypic plasticity, competition for pollinators and other resources, and species replacement (13, 15, 18, 45). Our results thus invite speculation that phenological divergence may create a potential reproductive niche for heat-tolerant species in mid-summer, which may be conducive to invasion by nonnatives. In addition, warming also caused compression and expansion of the temporal reproductive periods of different species and altered reproductive overlap between species pairs. The suite of changes in phenology caused by climate change will likely create powerful selection pressure not only on plant species themselves (33) but possibly also on species at higher trophic levels that depend on these plants. Such multitrophic level interactions with changes in phenology have been observed for a phytophagous fly in the eastern United States (46), parasite–grouse interactions in Canada (47), and an Atlantic marine community (7). Thus, the climate-induced disruption of phenological patterns potentially initiates changes that over time might affect community organization and have far-reaching consequences for ecology and evolution.
Methods
The Experiment.
The experiment was conducted at the Kessler Farm Field Laboratory in McClain County, OK (lat 34°58′54′′N, long 97°31.14′′W), ≈500 m north of the experiment reported in ref. 48, in which the soil, vegetation, land use history, and weather of the site have been characterized. The experiment was designed primarily to examine ecosystem responses to a 1-year temperature anomaly in interaction with double precipitation. The warming and precipitation treatments began on February 20, 2003, and ended on the same day in 2004. Twenty 3 × 2-m plots were placed 1.5 m apart in two rows ≈3 m apart. Ten of the 20 plots were selected to have two 165 × 15 cm infrared heaters (Kalglo Electronics Inc., Bethlehem, PA) suspended at a height of 1.5 m above the ground. [As shown in a rigorous test (49), the infrared heaters do not generate any visible light to influence phenology.] The remaining 10 plots were each hung with two “dummy” heaters to simulate shading effects of the heaters. Five of both the warmed and unwarmed plots were selected to receive doubled precipitation by using a “rainfall collection pan,” which was an angled catchment the same size and shape as the plot that funneled water onto these plots with each rain event so that the amount of rainfall was doubled.
Air temperature was monitored hourly with automated thermocouple systems (Campbell Science Equipment, Logan, UT) at 15 cm above and at depths of 7.5, 22.5, 45, 75, and 105 cm in the soil. Relative to the temperature in control plots, the air temperature increased by 4.17°C in the warmed plots, decreased by 0.44°C in the double precipitation plots, and increased by 4.83°C in the warming plus doubled precipitation plots during the observation period (February 20, 2003, through November 16, 2003). The increase in temperature, which is at the upper range of the Intergovernmental Panel on Climate Change projection (50), was designed to generate a perturbation large enough to affect ecosystem processes. Soil moisture was measured with time-domain reflectometery (ESI Equipment, Victoria, BC, Canada) inserted into the middle of each plot with five segments 0–15, 15–30, 30–60, 60–90, and 90–150 cm below the soil surface. Over the observation period, soil moisture (% volume) in the surface 15 cm of soil averaged 19.93 ± 0.94 in the control, 21.30 ± 0.42 in the double precipitation treatment, 14.43 ± 0.44 in the warming treatment, and 16.36 ± 0.44 in the warming plus doubled precipitation treatment.
Phenological Observations.
Eighty-two plant species were present in the experimental plots. The 12 species monitored were dominant in the plant community at the site. Together, these species made up 70–80% of the relative cover and ≈90% of the biomass. As soon as buds were noticed on any plant in any plot for each species, collection of phenological data of flowering began for that species. Most species were scored weekly. Exceptions are Panicum, which was scored biweekly, and Cerastium, which was scored every 10 days. For species with the smallest and most numerous plants (Viola, Veronica, Cerastium, Plantago, Dichanthelium, and Bromus), every plant with buds, flowers, or fruit in two quarters of the plot was counted and given a phenological score based on the phenological stage of the oldest flower position on the plant. For Erigeron and Achillea, each flowering stage present on every plant (whether in ray- or disk-florets) in each plot was noted. For Panicum, Andropogon, Schizachyrium, and Ambrosia, 10 stems (if available) of each species in each plot were tagged and each phenological stage present on each plant was recorded. The scoring of phenological stages was modified from Price and Waser (11) and Dunne et al. (12). For forbs, reproductive phenology was divided into seven stages: F0, vegetative plants; F1, unopened buds; F2, open flowers; F3, old flowers (postanthesis); F4, initiated fruit; F5, expanding fruit; and F6, dehisced fruit. For grasses, five reproductive phenology stages were distinguished: G0, plants with flower stalks (in boot); G1, spikelets present (out of boot); G2, exerted anthers or styles; G3, past the presence of anthers and styles (seed development); and G4, disarticulating florets. For forb species with very small flowers and fruits that were difficult to observe, stage 3 (initiated fruit) and stage 4 (expanding fruit) were lumped into a category of “fruit present,” (i.e., a score of F4.5). Because buds were formed long before they were visible in grasses, the beginning of the reproductive phase in grasses was taken to be the date when the most culms in boot were visible per plot and called G0.5. In the summer grasses, many florets never developed seed. In these cases, seed development could not be used as an indicator of phenological stage, and G3 was assigned as the stage after the presence of anthers and styles as indicated by partially expanded glume tips and/or erect awns. Data collection ended when all plants of a species had reached a phenological stage of F6 for forbs or G4 in grasses, or when most of the fruits had dehisced and further seed dehiscence was occurring so slowly that there was no change in phenological stage for a period of 2 weeks. An exception was Ambrosia, where plants were clipped at ground level at the first sign of seed dehiscence to measure seed set and above-ground biomass (data not shown).
Data Analysis.
To reduce variability among individual observations of phenological stages, phenological data from each of the 12 species in each plot were fitted to the Richards growth equation (51) with the contraction-expansion algorithm (52). The Richards equation is more flexible than the logistic equation to describe different shapes of growth (or increment) data (51). The calibrated Richards equations were used to calculate the time of flowering (stage F2 for forbs or G2 for grasses) and the time of fruiting (stage F3.5 for forbs and G2.5 for grasses) for each species in each plot (for the first eight species) or for each plant observed in each plot (for the last four species). (For more details on the averaging of data within plots and the Richards equation, see SI Text.)
Statistical differences in the four parameters of the Richards equation, flowering time, fruiting time, duration of reproduction, and the length of the bud phase, flower phase, and fruit phase between treatments were tested by multivariate ANOVA for each species by using the general linear models procedure of SAS 8.01 (SAS Institute 2002, Cary, NC). Test results are shown in SI Table 2. Two-sample t tests were used to determine whether the differences between treatments in overlap between species pairs were significant (SI Table 4). The critical value of t was determined using n = 5 (plots), giving 8 df.
Supplementary Material
Acknowledgments
We thank D. Ackerly, S. Collins, L. Gough, D. Hui, D. Inuoye, S. Wan, and S. Hu, three anonymous reviewers, and the associate editor for constructive suggestions on the manuscript and N. Zerhbach and M. Talley for field assistance. This work was supported by National Science Foundation Integrated Research Challenges in Environmental Biology Grant DEB 0078325.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0605642104/DC1.
References
- 1.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TRC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 2.Peñuelas J, Filella I. Science. 2001;294:793–795. doi: 10.1126/science.1066860. [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C, Yohe G. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 4.Root TL, Price JT, Hall KR, Schnelder SH, Rosenzweig C, Pounds JA. Nature. 2003;241:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 5.Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR. Nature. 1997;386:698–702. [Google Scholar]
- 6.Slayback DA, Pinzon JE, Los SO, Tucker CJ. Global Change Biol. 2003;9:1–15. [Google Scholar]
- 7.Edwards M, Richardson AJ. Nature. 2004;430:881–884. doi: 10.1038/nature02808. [DOI] [PubMed] [Google Scholar]
- 8.Nemani RR, Keeling CD, Hashimoto H, Jolly WM, Piper SC, Tucker CJ, Myeni RB, Running SW. Science. 2003;300:1560–1563. doi: 10.1126/science.1082750. [DOI] [PubMed] [Google Scholar]
- 9.Keeling CD, Chin JFS, Whorf TP. Nature. 1996;382:146–149. [Google Scholar]
- 10.Wan S, Yuan T, Bowdish S, Wallace L, Russell SD, Luo YQ. Am J Bot. 2002;89:1843–1846. doi: 10.3732/ajb.89.11.1843. [DOI] [PubMed] [Google Scholar]
- 11.Price MV, Waser NM. Ecology. 1998;79:1261–1271. [Google Scholar]
- 12.Dunne JA, Harte J, Taylor KJ. Ecol Monogr. 2003;73:69–86. [Google Scholar]
- 13.Rathcke B, Lacey EP. Annu Rev Ecol Syst. 1985;16:179–214. [Google Scholar]
- 14.Heinrich B. Ecology. 1976;57:890–899. [Google Scholar]
- 15.Kochmer JP, Handel SN. Ecol Monogr. 1986;56:303–325. [Google Scholar]
- 16.Ashton PS, Givnish TJ, Appanah S. Am Nat. 1988;132:44–66. [Google Scholar]
- 17.Tebar FJ, Gil L, Llorens L. Plant Ecology. 2004;174:293–303. [Google Scholar]
- 18.Ackerly DD. Int J Plant Sci. 2003;164:S165–S184. [Google Scholar]
- 19.Tilman D, Lehman CL, Thomson KT. Proc Natl Acad Sci USA. 1997;94:1857–1861. doi: 10.1073/pnas.94.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooper DU. Ecology. 1998;79:704–719. [Google Scholar]
- 21.Henry M, Stevens H, Carson WP. Oikos. 2001;92:291–296. [Google Scholar]
- 22.Waser NM, Real L. Nature. 1979;281:670–672. [Google Scholar]
- 23.Chuine I, Beaubien EG. Ecol Lett. 2001;4:500–510. and correction (2002) 5:316. [Google Scholar]
- 24.Post E, Forchhammer MC, Stenseth NC, Callaghan TV. Proc R Soc London Ser B. 2001;268:15–23. doi: 10.1098/rspb.2000.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley NL, Leopold AC, Ross J, Huffaker W. Proc Natl Acad Sci USA. 1999;96:9701–9704. doi: 10.1073/pnas.96.17.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparks TH, Smithers RJ. Weather. 2002;57:157–166. [Google Scholar]
- 27.Menzel A. In: Phenology: An Integrative Environmental Science. Schwartz M, editor. Dordrecht, The Netherlands: Kluwer; 2003. pp. 319–329. [Google Scholar]
- 28.Fitter AH, Fitter RSR, Harris ITB, Williamson MH. Funct Ecol. 1995;9:55–60. and correction (2002) 16:543. [Google Scholar]
- 29.Menzel A. Intl J Biometeorol. 2000;44:76–81. doi: 10.1007/s004840000054. [DOI] [PubMed] [Google Scholar]
- 30.Peñuelas J, Filella I, Comas P. Global Change Biol. 2002;8:531–544. [Google Scholar]
- 31.Taylor BR, Garbary DJ. Rhodora. 2003;105:118–135. [Google Scholar]
- 32.Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kübler K, Bissolli P, Braslavská O, Briede A. Global Change Biol. 2006;12:1969–1976. [Google Scholar]
- 33.Etterson JR, Shaw RG. Science. 2001;294:151–154. doi: 10.1126/science.1063656. [DOI] [PubMed] [Google Scholar]
- 34.Goldman DA, Willson MF. Bot Rev. 1986;52:157–194. [Google Scholar]
- 35.Kliber A, Eckert CG. Ecology. 2004;85:1675–1687. [Google Scholar]
- 36.Veresoglou DS, Fitter AH. J Ecol. 1984;72:259–272. [Google Scholar]
- 37.Ashman T-L. Am Nat. 1994;144:300–316. [Google Scholar]
- 38.Fageria NK. J Plant Nutr. 2004;27:947–958. [Google Scholar]
- 39.Terabayashi S, Muramatsu I, Tokutani S, Ando M, Kitagawa E, Shigemori T, Date S, Fujime Y. J Japan Soc Hort Sci. 2004;73:324–329. [Google Scholar]
- 40.Galen C, Stanton ML. Am J Bot. 1991;78:978–988. [Google Scholar]
- 41.Kudo G, Suzuki S. Arct Antarct Alp Res. 2002;34:185–190. [Google Scholar]
- 42.Saavedra F, Inouye DW, Price MV, Harte J. Global Change Biol. 2003;9:885–894. [Google Scholar]
- 43.Campbell DR. Am Nat. 1991;137:713–737. [Google Scholar]
- 44.Cleland EE, Chiariello NR, Loarie SR, Mooney HA, Field CB. Proc Natl Acad Sci USA. 2006;103:13740–13744. doi: 10.1073/pnas.0600815103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bawa KS, Kand H, Grayum MH. Am J Bot. 2003;90:877–887. doi: 10.3732/ajb.90.6.877. [DOI] [PubMed] [Google Scholar]
- 46.Filchak KE, Roethele JB, Feder JL. Nature. 2000;407:739–742. doi: 10.1038/35037578. [DOI] [PubMed] [Google Scholar]
- 47.Cattadori IM, Haydon DT, Hudson PJ. Nature. 2005;433:737–741. doi: 10.1038/nature03276. [DOI] [PubMed] [Google Scholar]
- 48.Luo YQ, Wan SQ, Hui DF, Wallace LL. Nature. 2001;413:622–625. doi: 10.1038/35098065. [DOI] [PubMed] [Google Scholar]
- 49.Kimball BA. Global Change Biol. 2005;11:2041–2056. [Google Scholar]
- 50.Intergovernmental Panel on Climate Change. Climate Change 2001: Synthesis Report, Third Assessment Report of the Intergovernmental Panel on Climate Change. New York: Cambridge Univ Press; 2001. [Google Scholar]
- 51.Richards FJ. J Exp Bot. 1959;29:290–300. [Google Scholar]
- 52.Gu SL, Hui DF, Bian AH. J Biomath. 1998;13:426–434. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.