Abstract
The authors reviewed the recruitment of stroke-affected sibling pairs using a letter-based, proband-initiated contact strategy. The authors randomly sampled 99 proband enrollment forms (Phase 1) and randomly sampled 50 sibling reply cards (Phase 2). The sibling response rate was 30.6%, for a pedigree response rate of 58%. Of the siblings who replied, 96% authorized further contact. Median time from proband enrollment to pedigree DNA banking, which required 3+ probands, was 134 days.
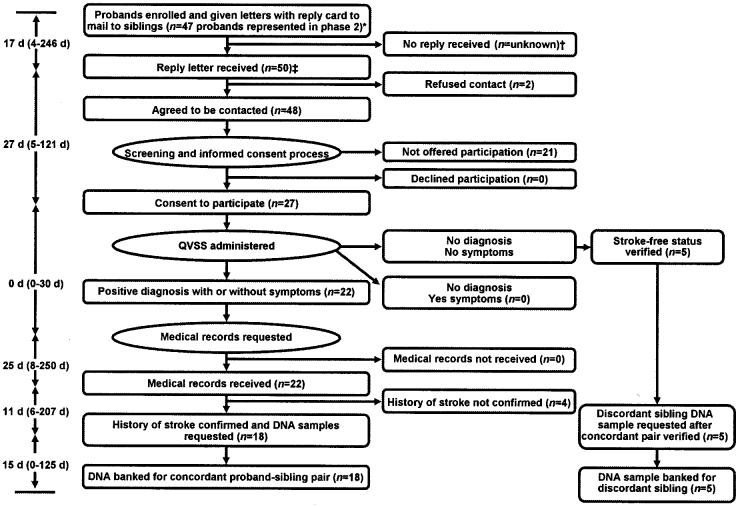
Enrolling pedigrees poses ethical and methodologic challenges.1-3 Recruitment strategies should be effective while ensuring privacy to family members. The Siblings with Ischemic Stroke Study (SWISS) is one of the first large-scale pedigree studies in North America specifically designed to investigate the genetics underlying the risk of ischemic stroke.4 SWISS uses proband-initiated contact by mail to recruit stroke-affected sibling pairs (figure 1). We reviewed the process from proband enrollment to pedigree DNA collection.
Figure 1.
Letter-based, proband-initiated contact process of the Siblings with Ischemic Stroke Study.
Methods
The protocol for SWISS has been published.4 Phase 1 of the current study was designed to assess the effectiveness of proband-initiated contact for recruiting siblings. Phase 2 was designed to assess the willingness of siblings to participate in the study and to determine the time required for each step in pedigree enrollment. We also calculated the number of probands needed to enroll (PNE) to complete one pedigree.
In Phase 1, we reviewed a random sample of 99 proband enrollment forms. Date of enrollment (the date that the consent form was signed) and proband study number were abstracted from each form. We then reviewed every sibling response associated with each proband. We recorded the number of sibling responses, the number of positive and negative authorizations for contact by researchers, and dates that reply card responses were received.
In Phase 2, we reviewed an independent random sample of 50 sibling replies. We determined whether the sibling expressed an interest in participating. For those who did, we determined whether a signed consent form was obtained. For siblings who gave consent, we recorded their stroke status as determined by the Questionnaire to Verify Stroke-free Status (QVSS).5-7 For potentially stroke-affected (concordant) siblings, we recorded the outcomes of the records review and the request for blood samples. We also recorded the date that each step was completed.
For PNE calculation, we considered a pedigree complete when blood samples were obtained from the proband and one concordant sibling. We also recorded the number of samples from discordant siblings.
Results
By May 1, 2003, 371 probands had been enrolled in SWISS from 49 actively enrolling centers. DNA samples (N = 264) from 112 pedigrees had been obtained and stored from 112 probands, 112 concordant siblings, and 40 discordant siblings.
Phase 1
We sampled 99 of 371 probands. One proband was assigned a randomization number but withdrew consent before letters of invitation were mailed to siblings. The remaining 98 probands had 343 living siblings who presumably received letters. The reported number of siblings per proband was one to 12 (median, 3) (table).
Table.
Response by sibship size
| No. of pedigrees |
|||||
|---|---|---|---|---|---|
| Reported no. of siblings | Total* | With no reply | With at least one reply | Completely captured | Ratio of responding siblings to reported siblings |
| 1 | 25 | 8 | 17 | 17 | 0.68 |
| 2 | 13 | 3 | 10 | 6 | 0.62 |
| 3 | 20 | 8 | 12 | 1 | 0.28 |
| 4 | 15 | 9 | 6 | 2 | 0.25 |
| 5 | 7 | 4 | 3 | 0 | 0.17 |
| 6 | 5 | 1 | 4 | 1 | 0.67 |
| 7 | 6 | 3 | 3 | 0 | 0.24 |
| ≥8 | 7 | 6 | 1 | 0 | 0.06 |
| Total | 98 | 41 | 57 | 27 | 0.31 |
One proband withdrew before mailing any sibling letters.
We received 105 reply cards. The median number of sibling responses per proband was one (range 0 to 6). Of siblings who responded, 101 (96%) authorized contact (representing 55 pedigrees). Four siblings (4%) from four different pedigrees requested no further contact; two pedigrees had another sibling who authorized contact. No reply was received from 238 siblings (representing 41 pedigrees). The response profile for all 343 sibling letters given to probands to mail was 29.4% (101) for those who actively agreed to further contact, 1.2% (4) for those who actively refused further contact, and 69.4% (238) for those who did not respond. The within-pedigree response was 0% to 100% (median, 33.3%). Blood samples from 26 distinct pedigrees were collected from these 98 probands (26 concordant sibling pairs and 11 discordant siblings).
Phase 2
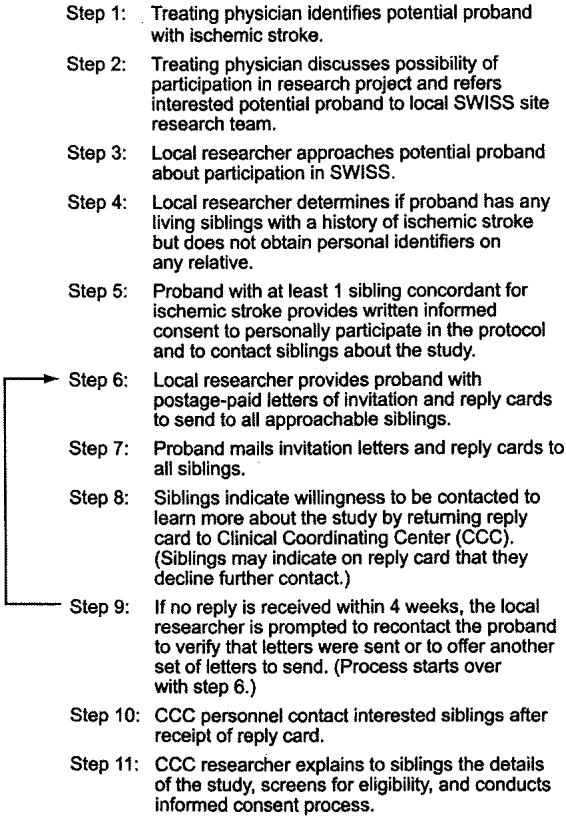
For the random sample of 50 siblings who returned reply cards to the clinical coordinating center, responses were received between January 23, 2001, and April 8, 2003. These siblings were traced through enrollment events from receipt of reply cards to obtainment of blood samples (figure 2). The 50 reply cards came from 47 separate sibships. Of the 50 siblings who returned reply cards, two siblings (4%) declined further contact and 48 (96%) allowed contact.
Figure 2.
Sibling recruitment and enrollment in the Siblings with Ischemic Stroke Study (SWISS). Numbers correspond to data from Phase 2 of the study. Intervals at left are median (range) number of days for each step. QVSS, Questionnaire to Verify Stroke-free Status (a validated structured interview used in SWISS). *Because these data were derived from a sample of 50 sibling reply cards, the number of probands was back-calculated. †Data for sibship size in Phase 2 were not available. ‡From a random sample of 50 sibling reply cards used in Phase 2.
Of the 48 siblings who responded affirmatively, 27 were enrolled. Of the 21 who were not enrolled, five were extra discordant siblings, three had no concordant sibling enrolled, two had a concordant sibling who withdrew consent, and one had a proband who died. One proband and one concordant sibling were later determined not to have had an ischemic stroke, so no siblings could be enrolled. One sibling was a member of a previously enrolled pedigree (double ascertainment). Seven siblings had no identifiable reason for not enrolling.
Of the 27 enrolled siblings, 22 had a QVSS-verified history of stroke and five were verified as stroke free (discordant). Medical records of the 22 potential concordant siblings confirmed 18 strokes. DNA was collected from 18 concordant sibling pairs and five discordant siblings. The median time from proband enrollment to pedigree DNA banking was 134 days.
PNE
For SWISS overall, we ascertained 371 probands and completed a total of 112 pedigrees with DNA collected (3.3 PNEs for one pedigree). In Phase 1, from 99 probands, we obtained blood from 26 concordant sibling pairs and 11 discordant siblings (PNE 3.8). Restricting the calculation to only the 57 probands for whom a sibling reply card was received reduced the PNE to 2.2. Similarly, in Phase 2, reply cards were received from siblings of 47 probands, and DNA was obtained from 22 distinct pedigrees, for a PNE of 2.1.
Discussion
Proband-sibling pairs with a late-life morbid disease can be enrolled in genetic research by a letter-based, proband-initiated contact strategy. Yet, we observed a substantial decrease in potentially eligible siblings from proband enrollment to sibling reply. Using letter-based, proband-initiated contact resulted in a sibling response rate of 30.6%, for a pedigree response rate of 58%. Of siblings who replied, a high percentage authorized further contact: 101 of 105 (96.2%) siblings and 55 of 57 (96.5%) pedigrees. Interpreting the high nonresponse rate of 69.4% (238 of 343 reported siblings representing 41 of 98 pedigrees) is challenging. Letter-based, proband-initiated contact does not allow for distinguishing whether nonresponders preferred not to participate (passive decliners), did not receive the information, or had not read the invitation to participate. We also suspect that disease-related characteristics, such as age and poor health, may contribute to the nonresponse rate.
The PNE statistic might be helpful for comparing pedigree recruitment strategies. We found the PNE from proband identification to DNA banking to be 3.3 to 3.8. Thus, three of four probands have not yet been approached for DNA sample collection because a concordant sibling has yet to be enrolled. For two of these three, the likely reason is the nonresponse of siblings.
Letter-based, proband-initiated contact achieves its intended purpose of protecting privacy but at a cost of inefficient recruitment. The goals of protecting privacy and minimizing intrusion into the lives of siblings might be achieved by having probands obtain oral permission from siblings to give their contact information to researchers.8 This method shifts some of the burden of establishing contact with siblings to the research team, allows rapid ascertainment of a definitive response, permits the targeting of initial efforts at concordant siblings, decreases the proportion of nonresponders, and allows a more rapid attainment of the proband-sibling pair. We hypothesize that this approach may also decrease the number of PNEs compared with letter-based, proband-initiated contact.
Another strategy includes obtaining permission only from probands before contacting family members. This strategy was used successfully during the SWISS pilot study in which the PNE was 1.5.9 Although more efficient, this approach was abandoned in SWISS because of evolving standards of privacy protection in the United States and because of differing institutional review board positions on whether the provision of sibling names by probands makes the siblings passive research subjects.
Appendix
SWISS participating centers as of July 1, 2004. (Asterisk indicates site closed to enrollment.)
Probands enrolled: 47. Mayo Clinic, Rochester, MN. Principal investigator: Robert D. Brown, Jr., MD; subinvestigators: George Petty, MD; Eelco Wijdicks, MD; Irene Meissner, MD; Bruce Evans, MD; Kelly Flemming, MD; Edward Manno, MD; Jimmy Fulgham, MD; David Wiebers, MD; coordinator: Colleen Albers, RN.
Probands enrolled: 37. University of Florida/Shands Jacksonville, Jacksonville. Principal investigator: Scott L. Silliman, MD; coordinators: Barbara Quinn, RN; Cicely Bryant.
Probands enrolled: 33. Mayo Clinic, Jacksonville, FL. Principal investigator: Thomas G. Brott, MD; subinvestigators: James F. Meschia, MD; Frank Rubino, MD; Benjamin Eidelman, MD; coordinator: Jacob Rosenberg, CRC.
Probands enrolled: 31. Mercy Ruan Neurology Clinic and Clinical Research Center, Des Moines, IA. Principal investigator: Michael Jacoby, MD; subinvestigators: Bruce Hughes, MD; Randall Hamilton, MD; Paul Babikian, MD; Mark Puricelli, DO; coordinator: Judi Greene, RN.
Probands enrolled: 29. University of Cincinnati, Cincinnati, OH. Principal investigator: Brett M. Kissela, MD; subinvestigators: Joseph Broderick, MD; Daniel Woo, MD; Daniel Kanter, MD; Dawn Kleindorfer, MD, Alexander Schneider, MD; Matthew Flahery, MD; coordinator: Kathleen Alwell, RN.
Probands enrolled: 29. University of Virginia, Charlottesville. Principal investigator: Bradford B. Worrall, MD, MSc; subinvestigators: E. Clarke Haley, Jr., MD; Karen Johnston, MD, MSc; Jaclyn van Wingerden, BBA; coordinator: Martha Davis, RN.
Probands enrolled: 20. Neurologic Associates, Inc., Richmond, VA. Principal investigator: Francis E. McGee, Jr., MD; subinvestigators: Stephen Thurston, MD; Thomas Smith, MD; Robert White, MD; Philip Davenport, MD; John Brush, MD; Susanna Mathe, MD; Robert Cohen, MD; J. Kim Harris, MD; John O'Bannon III, MD; John Blevins, MD; coordinators: Sharon McQueen-Goss, RN; Janet McGee, REPT, CCRC.
Probands enrolled: 15. Mercy General Hospital, Sacramento, CA. Principal investigator: Paul T. Akins, MD, PhD; coordinator: Deidre Wentworth, RN.
Probands enrolled: 13. Maine Line Health—Stroke Program, Bryn Mawr, PA. Principal investigator: Gary H. Friday, MD, MPH; coordinator: Angela Whittington-Smith, RN.
Probands enrolled: 12. Centre Hospitalier Affilié Universitaire de Québec, Quebec City, Province of Quebec, Canada. Principal investigator: Denis Simard, MD; subinvestigator: Ariane Mackey, MD; coordinators: Annette Hache, RN; Sophie Dube, RN.
Probands enrolled: 11. Luther Midelfort Clinic, Eau Claire, WI. Principal investigator: Felix Chukwudelunzu, MD; subinvestigators: James Bounds, MD; Rae Hanson, MD; David Nye, MD; Donn Dexter, MD; coordinators: Tonya Kunz, RN; Karen Snobl, RN.
Probands enrolled: 11. Maine Medical Center, Portland, ME. Principal investigator: John R. Belden, MD; subinvestigator: Paul Muscat, MD; coordinator: Diane Diconzo-Fanning, RN.
Probands enrolled: 11. Wake Forest University School of Medicine, Winston-Salem, NC. Principal investigator: David Lefkowitz, MD; subinvestigators: Charles Tegeler IV, MD; Patrick Reynolds, MD; coordinators: Jean Satterfield, RN; Elizabeth Westerberg, CCRC.
Probands enrolled: 10. University of South Alabama, Mobile. Principal investigator: Richard Zweifler, MD; subinvestigators: Ivan Lopez, MD; M. Asim Mahmood, MD; coordinators: Robin Yunker, RNC, MSN; Mel Parnell, RN, BSN.
Probands enrolled: 9. Cleveland Clinic Florida, Weston, FL. Principal investigator: Virgilio Salanga, MD; subinvestigators: Eduardo Locatelli, MD; Nestor Galvez-Jimenez, MD, FACP; Efrain Salgado, MD; coordinators: Anupama Podichetty, MD; Jose Alvarez, MD.
Probands enrolled: 9. University of Pennsylvania Medical Center, Philadelphia. Principal investigator: Scott E. Kasner, MD; subinvestigators: David S. Liebeskind, MD; Brett L. Cucchiara, MD; Michael L. McGarvey, MD; Steven R. Messe, MD; Robert A. Taylor, MD; coordinator: Jessica Clarke, RN.
Probands enrolled: 8. Washington University School of Medicine, St. Louis, MO. Principal investigator: Jin-Moo Lee, MD, PhD; subinvestigator: Abdullah Nassief, MD; coordinator: Denise Shearrer, RMA, BS.
Probands enrolled: 8. Hospital Charles LeMoyne, Greenfield Park, Province of Quebec, Canada. Principal investigator: Leo Berger, MD; coordinators: Martine Maineville; Denise Racicot.
Probands enrolled: 7. Emory University School of Medicine, Atlanta, GA. Principal investigator: Barney J. Stern, MD; subinvestigators: Michael Frankel, MD; Marc Chimowitz, MD; Owen Samuels, MD; coordinator: Betty Jo Shipp, RN.*
Probands enrolled: 7. Mayo Clinic, Scottsdale, AZ. Principal investigator: David W. Dodick, MD; subinvestigator: Bart Demaerschalk, MD; coordinators: Erica Boyd, RN; Rebecca Rush, RN; Gail LeBrun, RN; Nadine Lendzion, RN; Barbara Cleary, RN.
Probands enrolled: 7. University of California–Davis School of Medicine, Sacramento. Principal investigator: Piero Verro, MD; coordinator: Shari Nichols.
Probands enrolled: 7. University of Iowa Hospital, Iowa City. Principal investigator: Patricia Davis, MD; subinvestigator: Harold P. Adams, Jr., MD; coordinator: Jeri Sieren, RN.
Probands enrolled: 7. University of Texas Southwestern Medical Center at Dallas. Principal investigator: D. Hal Unwin, MD; subinvestigators: Dion Graybeal, MD; Mark Johnson, MD; Mounzer Kassab, MD; coordinator: J. Greggory Wright, BS.
Probands enrolled: 7. University of Wisconsin, Madison. Principal investigator: Robert Dempsey, MD; subinvestigators: George Newman, MD; Douglas Dulli, MD; Madeleine Geraghty, MD; coordinator: Pam Winne.
Probands enrolled: 7. MetroHealth Medical Center, Cleveland, OH. Principal investigator: Joseph P. Hanna, MD; subinvestigators: Marc Winkelman, MD; Nimish Thakore, MD, DM; coordinators: Alice Liskay, RN; Joan Kappler, RN; Dana Simcox, RN.
Probands enrolled: 7. Kaleida Stroke Center—Millard Fillmore Hospital, Buffalo, NY. Principal investigator: F. E. Munschauer, MD; subinvestigator: Peterkin Lee-Kwen, MD; coordinator: Kathleen Wrest, MLS.
Probands enrolled: 7. Stroke Prevention and Atherosclerosis Research Centre (SPARC), Roberts Research Institute, London, Ontario, Canada. Principal investigator: J. David Spence, MD; subinvestigator: Claudio Munoz, MD; coordinator: Rose Freitas.
Probands enrolled: 6. East Bay Region Associates in Neurology, Berkeley, CA. Principal investigator: Brian Richardson, MD; coordinator: Lauren McCormick.
Probands enrolled: 6. Indiana University School of Medicine, Indianapolis, IN. Principal investigator: Linda S. Williams, MD; subinvestigators: Askiel Bruno, MD; William Jones, MD.
Probands enrolled: 5. Helen Hayes Hospital, West Haverstraw, NY. Principal investigator: Laura Lennihan, MD; coordinator: Laura Tenteromano, RN.
Probands enrolled: 5. Thomas Jefferson University Hospital, Philadelphia. Principal investigator: Rodney D. Bell, MD; subinvestigators: David G. Brock, MD; Carissa Pineda, MD; coordinator: Lisa Bowman, MNS, CRNP, CNRN.
Probands enrolled: 5. Ohio State University, Columbus. Principal investigator: Andrew P. Slivaka, Jr., MD; subinvestigator: Yousef Mohammad, MD; coordinator: Peggy Notestine, CCRC.
Probands enrolled: 5. Inova Fairfax Hospital, Falls Church, VA. Principal investigator: Paul Nyquist, MD; coordinator: Barbara Farmer, RN, MSN.
Probands enrolled: 4. Florida Neurovascular Institute, Tampa. Principal investigator: Erfan A. Albakri, MD; coordinators: Taryn Chauncey, RN; Judy Jackson; Mary Katherine Taylor, ARNP.
Probands enrolled: 4. Marshfield Clinic, Marshfield, WI. Principal investigator: Percy N. Karanjia, MD; subinvestigator: Kenneth Madden, MD; coordinator: Kathy Mancl, CCRC.
Probands enrolled: 4. University of Kentucky, Lexington. Principal investigator: L. Creed Pettigrew, MD; subinvestigators: Stephen Ryan, MD; Anand G. Vaishnav, MD; coordinator: Deborah Taylor, MS.
Probands enrolled: 4. University of Maryland, Baltimore. Principal investigator: Steven J. Kittner, MD, MPH; subinvestigator: John Cole, MD; coordinator: Mary J. Sparks, RN, BSN.
Probands enrolled: 3. Field Neurosciences Institute, Saginaw, MI. Principal investigator: Faith Abbott, MD; subinvestigators: Malcolm Field, MD; Debasish Mridha, MD; coordinator: Richard Herm, RN, BSN, CEN.
Probands enrolled: 3. Johns Hopkins Bayview Medical Center, Baltimore. Principal investigator: Rafael H. Llinas, MD; subinvestigator: Christopher Earley, MD; coordinator: Janice Alt, RN, BSN.*
Probands enrolled: 3. Medical University of South Carolina, Charleston. Principal investigator: Timothy D. Carter, MD.
Probands enrolled: 3. Royal University Hospital, Saskatoon, Saskatchewan, Canada. Principal investigator: Ali H. Rajput, MD; subinvestigator: Alexander Rajput, MD; coordinator: Theresa Shirley, RN.*
Probands enrolled: 3. Rush-Presbyterian-St. Luke's Medical Center, Chicago. Principal investigator: Sean Ruland, DO; subinvestigators: Michael Schneck, MD; Michael Sloan, MD; Phillip Gorelick, MD, MPH; coordinator: Karen Whited, RN.*
Probands enrolled: 3. Scripps Clinic, La Jolla, CA. Principal investigator: Mary A. Kalafut, MD; coordinators: Carmen James, RN; Joy Reyes.
Probands enrolled: 3. University of California Los Angeles Stroke Center. Principal investigator: Jeffery L. Saver, MD; subinvestigators: Bruce Ovbiagele, MD; Scott Selco, MD; Venkatakrishna Rajajee, MD; coordinator: Gina Paek, BA.
Probands enrolled: 3. University of California San Diego Stroke Center. Principal investigator: Patrick D. Lyden, MD; subinvestigators: Christy Jackson, MD; Thomas Hemmen, MD; Brett Meyer, MD; coordinators: Nancy Kelly, RN; Janet Werner, RN.*
Probands enrolled: 3. University of Illinois at Chicago. Principal investigator: Cathy Helgason, MD; coordinator: Joan N. Martellotto, RN, PhD.*
Probands enrolled: 2. Chattanooga Neurology Associates, Chattanooga, TN. Principal investigator: Thomas Devlin, MD, PhD; subinvestigators: Adele Ackell, MD; Sharon Farber, MD; Ravi Chander, MD; G. Hagan Jackson, MD; Kadrie Hytham, MD; Bruce Kaplan, MD; David Rankine, MD; coordinators: Patty Wade-Hardie, RN; Tammy Owens, RN.*
Probands enrolled: 2. Yale University School of Medicine, New Haven, CT. Principal investigator: Mark Gorman, MD; coordinator: Janet Halliday, RN, BS.
Probands enrolled: 1. Charles R. Drew University of Medicine and Science, Los Angeles. Principal investigator: George Locke, MD; subinvestigator: Lowell Nelson, PhD; coordinators: Marcia Montenegro, RN; Derek Knight.
Probands enrolled: 1. Rhode Island Hospital, Providence. Principal investigator: Janet Wilterdink, MD; coordinator: Carol Cirillo, RN.*
Probands enrolled: 1. Syracuse VA Medical Center, Syracuse, NY. Principal investigator: Antonio Culebras, MD; coordinator: Therese Dean, RN.*
Footnotes
SWISS is supported by grant R01NS39987 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (J.F.M., Principal Investigator).
Data from this paper were presented at the 56th American Academy of Neurology Annual Meeting in San Francisco, April 27, 2004.
References
- 1.Worrall BB, Chen DT, Meschia JF. Ethical and methodological issues in pedigree stroke research. Stroke. 2001;32:1242–1249. doi: 10.1161/01.str.32.6.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botkin J. Protecting the privacy of family members in survey and pedigree research. JAMA. 2001;285:207–211. doi: 10.1001/jama.285.2.207. [DOI] [PubMed] [Google Scholar]
- 3.Chen DT, Worrall BB, Meschia JF. Protecting the privacy of family members in research. JAMA. 2001;285:1961–1963. [PubMed] [Google Scholar]
- 4.Meschia JF, Brown RD, Jr., Brott TG, Chukwudelunzu FE, Hardy J, Rich SS. The Siblings with Ischemic Stroke Study (SWISS) protocol. BMC Med Genet. 2002;3:1. doi: 10.1186/1471-2350-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meschia JF, Brott TG, Chukwudelunzu FE, et al. Verifying the stroke-free phenotype by structured telephone interview. Stroke. 2000;31:1076–1080. doi: 10.1161/01.str.31.5.1076. [DOI] [PubMed] [Google Scholar]
- 6.Jones WJ, Williams LS, Meschia JF. Validating the Questionnaire for Verifying Stroke-Free Status (QVSFS) by neurological history and examination. Stroke. 2001;32:2232–2236. doi: 10.1161/hs1001.096191. [DOI] [PubMed] [Google Scholar]
- 7.Meschia JF, Lojacono MA, Miller MJ, Brott TG, Atkinson EJ, O'Brien PC. Reliability of the questionnaire for verifying stroke-free status. Cerebrovasc Dis. 2004;17:218–223. doi: 10.1159/000075794. [DOI] [PubMed] [Google Scholar]
- 8.Kaye WH, Lilenfeld LR, Berrettini WH, et al. A search for susceptibility loci for anorexia nervosa: methods and sample description. Biol Psychiatry. 2000;47:794–803. doi: 10.1016/s0006-3223(99)00240-1. [DOI] [PubMed] [Google Scholar]
- 9.Meschia JF, Brott TG, Hardy J, et al. the SWISS Pilot Investigators Genome-wide screen for stroke: pilot testing in the Siblings with Ischemic Stroke Study (SWISS) J Stroke Cerebrovasc Dis. 2000;9:276–281. [Google Scholar]