Abstract
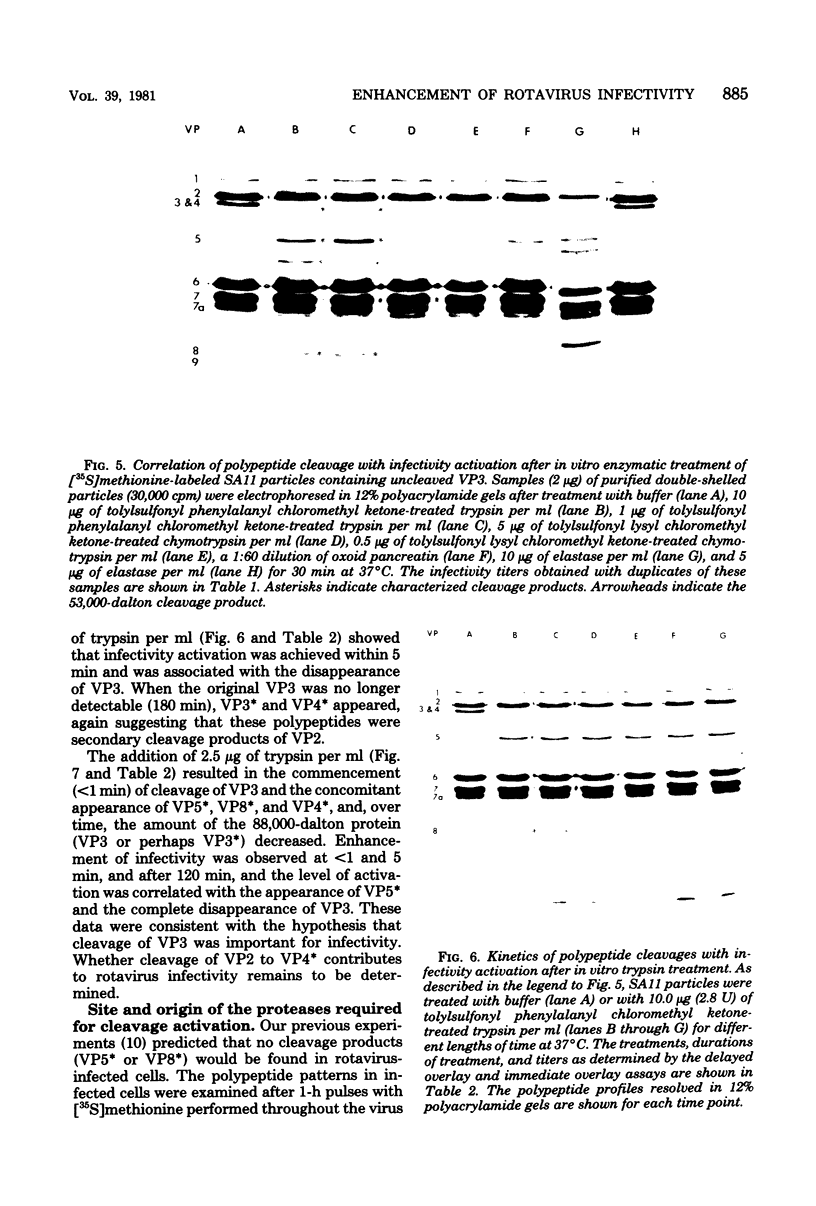
The polypeptide compositions of single-shelled and double-shelled simian rotavirus particles were modified by exposure to proteolytic enzymes. Specifically, a major outer capsid polypeptide (VP3) having a molecular weight of 88,000 in double-shelled particles was cleaved by trypsin to yield two polypeptides, VP5* and VP8* (molecular weights, 60,000 and 28,000, respectively). The cleavage of VP3 by enzymes that enhanced infectivity (trypsin, elastase, and pancreatin) yielded different products compared to those detected when VP3 was cleaved by chymotrypsin, which did not enhance infectivity. The appearance of VP5* was correlated with an enhancement of infectivity. Cleavages of the major internal capsid polypeptide VP2 were also observed. The VP2 cleavage products had molecular weights similar to those of known structural and nonstructural rotavirus polypeptides. We confirmed the precursor-product relationships by comparing the peptide maps of the polypeptides generated by digestions with V-8 protease and chymotrypsin. The remaining rotavirus structural polypeptides, including the outer capsid glycoproteins (VP7 and 7a), were not altered by exposure to pancreatic enzymes. Cleavage of VP3 was not required for virus assembly, and specific cleavage of the polypeptides occurred only on assembled particles. We also discuss the role of cleavage activation in other virus-specific biological functions (e.g., hemagglutination and virulence).
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D., Hall T., Banatvala J. E., Totterdell B. M., Chrystie I. L. The effect of trypsin on the growth of rotavirus. J Gen Virol. 1978 Jul;40(1):213–218. doi: 10.1099/0022-1317-40-1-213. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Mohammed K., Spence L., Fauvel M., Petro R. Rotavirus isolation and cultivation in the presence of trypsin. J Clin Microbiol. 1977 Dec;6(6):610–617. doi: 10.1128/jcm.6.6.610-617.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett B. B., Spendlove R. S., Clark M. L. Effect of enzymes on rotavirus infectivity. J Clin Microbiol. 1979 Jul;10(1):111–113. doi: 10.1128/jcm.10.1.111-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C., Woode G. N. Characterization of two particle types of calf rotavirus. J Gen Virol. 1976 May;31(2):245–250. doi: 10.1099/0022-1317-31-2-245. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Clark S. M., Barnett B. B., Spendlove R. S. Production of high-titer bovine rotavirus with trypsin. J Clin Microbiol. 1979 Mar;9(3):413–417. doi: 10.1128/jcm.9.3.413-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., López S., Arias C. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. J Virol. 1981 Jan;37(1):156–160. doi: 10.1128/jvi.37.1.156-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Estes M. K. Proteolytic enhancement of rotavirus infectivity: biology mechanism. Virology. 1980 Mar;101(2):432–439. doi: 10.1016/0042-6822(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Kalica A. R., Wyatt R. G., Jones R. W., Kapikian A. Z., Chanock R. M. Rescue of noncultivatable human rotavirus by gene reassortment during mixed infection with ts mutants of a cultivatable bovine rotavirus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):420–424. doi: 10.1073/pnas.78.1.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes E. C., Lee P. W., Miller S. E., Joklik W. K. The interaction of a series of hybridoma IgGs with reovirus particles. Demonstration that the core protein lambda 2 is exposed on the particle surface. Virology. 1981 Jan 15;108(1):147–155. doi: 10.1016/0042-6822(81)90534-1. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Theodore T. S. Polypeptides of simian rotavirus (SA-11) determined by a continuous polyacrylamide gel electrophoresis method. J Gen Virol. 1979 May;43(2):463–466. doi: 10.1099/0022-1317-43-2-463. [DOI] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Mukoyama A. Polypeptides of bovine rotavirus. J Gen Virol. 1979 May;43(2):309–316. doi: 10.1099/0022-1317-43-2-309. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F., Bridger J. C., Woode G. N. Characterisation of a rotavirus.20b. Nature. 1975 Dec 18;258(5536):631–633. doi: 10.1038/258631a0. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Palmer E. L., Martin M. L. Biochemical characterization of infantile gastroenteritis virus (IGV). J Gen Virol. 1977 Mar;34(3):485–497. doi: 10.1099/0022-1317-34-3-485. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J Virol. 1975 Nov;16(5):1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Further biochemical characterization, including the detection of surface glycoproteins, of human, calf, and simian rotaviruses. J Virol. 1977 Oct;24(1):91–98. doi: 10.1128/jvi.24.1.91-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. L., Lazdins I., Holmes I. H. Coding assignments of double-stranded RNA segments of SA 11 rotavirus established by in vitro translation. J Virol. 1980 Mar;33(3):976–982. doi: 10.1128/jvi.33.3.976-982.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil K. W., Bohl E. H., Agnes A. G. Cell culture propagation of porcine rotavirus (reovirus-like agent). Am J Vet Res. 1977 Nov;38(11):1765–1768. [PubMed] [Google Scholar]
- Thouless M. E. Rotavirus polypeptides. J Gen Virol. 1979 Jul;44(1):187–197. doi: 10.1099/0022-1317-44-1-187. [DOI] [PubMed] [Google Scholar]
- Todd D., McNulty M. S. Biochemical studies on a reovirus-like agent (rotovirus) from lambs. J Virol. 1977 Mar;21(3):1215–1218. doi: 10.1128/jvi.21.3.1215-1218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]