Abstract
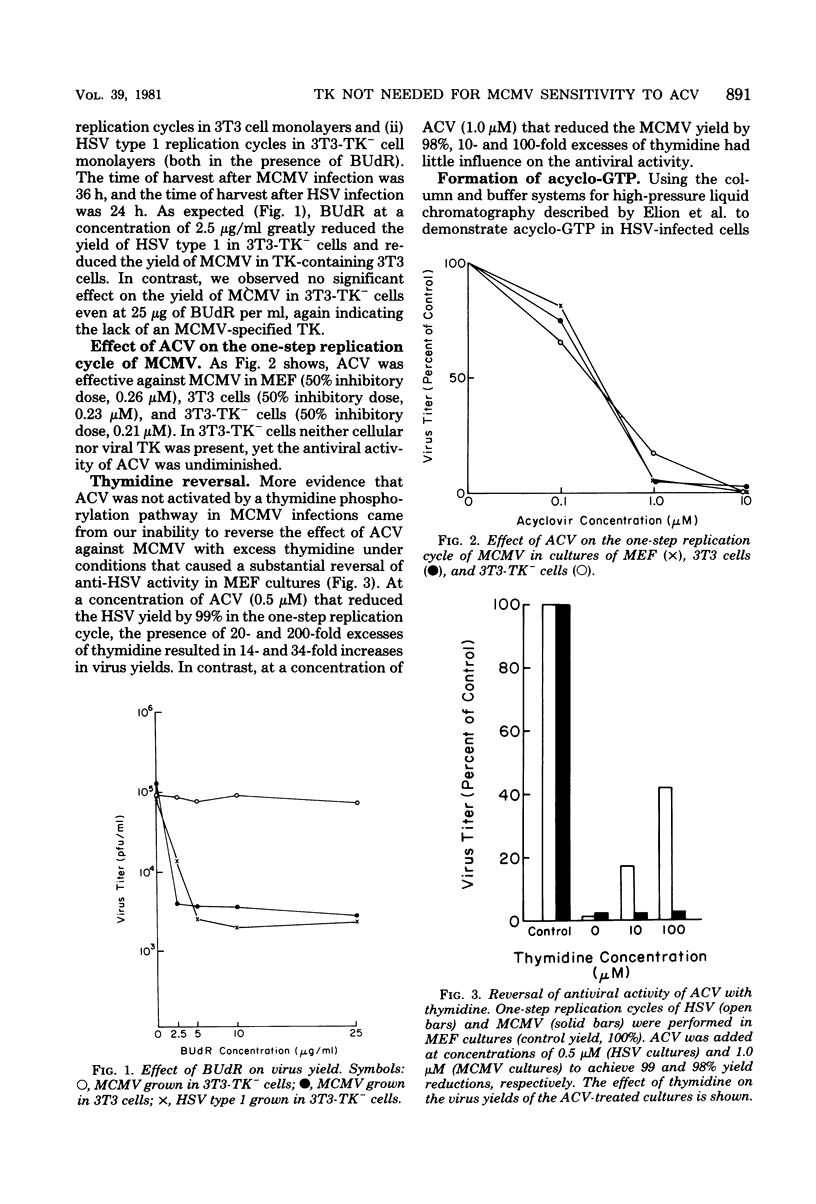
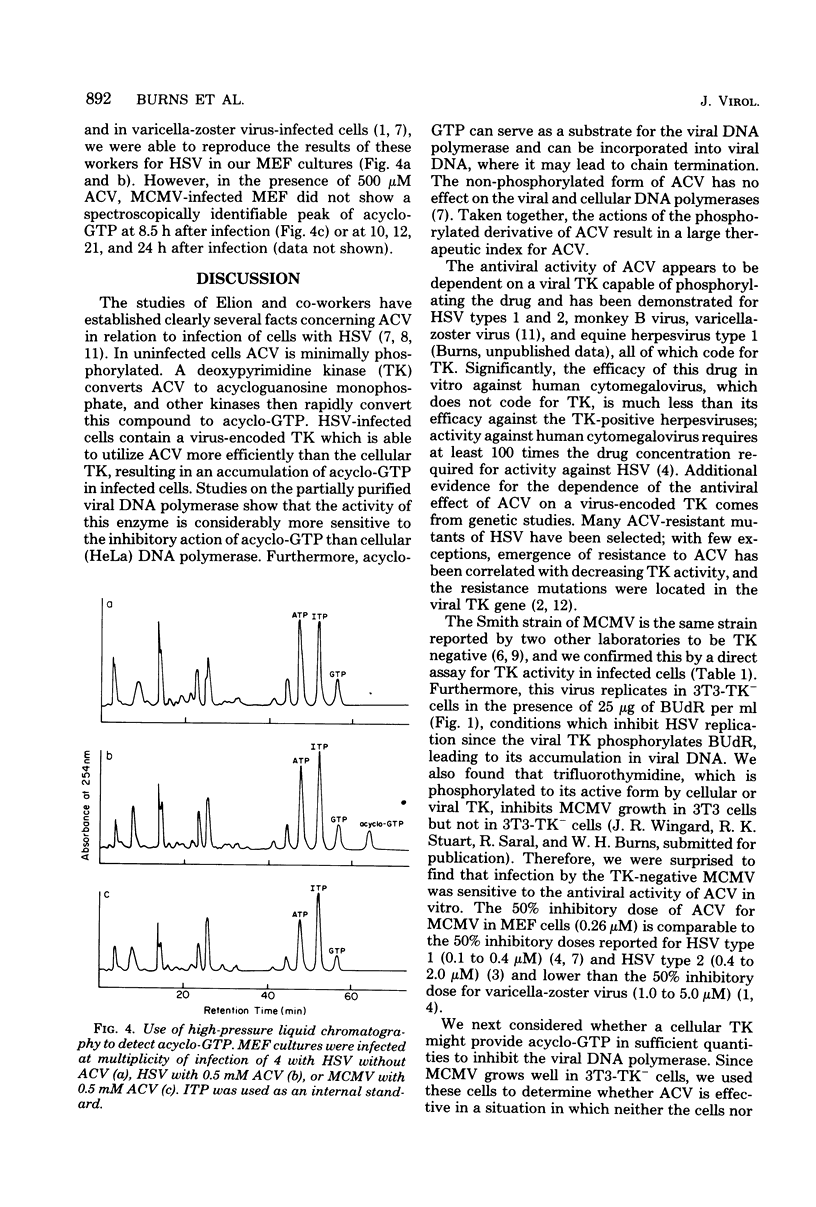
Previous studies of herpesvirus infections have indicated that a virus-specified thymidine kinase is required for the initial phosphorylation of acyclovir [acycloguanosine or 9-(2-hydroxyethoxymethyl)guanine] in the formation of acycloguanosine triphosphate. The latter compound accumulates in infected cells and competitively inhibits the viral DNA polymerase. We found that mouse cytomegalovirus, which does not express a thymidine kinase, was sensitive to the antiviral effects of acyclovir at a 50% inhibitory dose of approximately 0.23 microM. Acyclovir was equally effective against mouse cytomegalovirus in normal 3T3 cells and in 3T3 cells deficient in cellular thymidine kinase. Furthermore, the activity of acyclovir could not be reversed by excess thymidine, which easily reversed the antiviral activity of acyclovir against herpes simplex virus. Using a high-pressure liquid chromatography technique that easily detected acycloguanosine triphosphate in cells infected with herpes simplex virus, we could not detect acycloguanosine triphosphate in mouse cytomegalovirus-infected cells. These experiments demonstrated that the activity of acyclovir against mouse cytomegalovirus is not dependent on a thymidine phosphorylation pathway. Additional experiments are underway to determine whether acycloguanosine triphosphate is produced by another pathway in concentrations sufficient to inhibit mouse cytomegalovirus DNA polymerase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby B. M., Shaw J. E., Elion G. B., Pagano J. S. Effect of acyclovir [9-(2-hydroxyethoxymethyl)guanine] on Epstein-Barr virus DNA replication. J Virol. 1980 May;34(2):560–568. doi: 10.1128/jvi.34.2.560-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Zaia J. A., Levin M. J. Growth inhibition by acycloguanosine of herpesviruses isolated from human infections. Antimicrob Agents Chemother. 1979 May;15(5):642–645. doi: 10.1128/aac.15.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Colby B. M., Shaw J. E., Pagano J. S. Acyclovir inhibition of Epstein-Barr virus replication. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5163–5166. doi: 10.1073/pnas.77.9.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizuru Y., Minamishima Y., Hirano A., Kurimura T. Replication of mouse cytomegalovirus in thymidine kinase-deficient mouse cells. Microbiol Immunol. 1978;22(12):755–764. doi: 10.1111/j.1348-0421.1978.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Fyfe J. A., Rideout J. L., Keller P. M., Elion G. B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979 Oct;32(1):72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T., Hudson J. B. Thymidine kinase activity in mouse 3T3 cells infected by murine cytomegalovirus (MCV). Virology. 1977 Jul 15;80(2):430–433. doi: 10.1016/s0042-6822(77)80019-6. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Cell-free synthesis of herpes simplex virus-coded pyrimidine deoxyribonucleoside kinase enzyme. J Virol. 1977 Sep;23(3):455–460. doi: 10.1128/jvi.23.3.455-460.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Schnipper L. E., Crumpacker C. S. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]


