Abstract
The electrophysiological correlates of recollection were investigated with a modified Remember/Know task in which subjects signaled whether they fully or partially recollected visual object information in each study episode. A positive-going ERP deflection - the left parietal old/new effect - was sensitive to the amount of information recollected, demonstrating greater amplitude when elicited by test items associated with full relative to partial recollection. These findings support prior proposals that the left parietal ERP old/new effect is sensitive to the amount of information recollected from episodic memory. An early-onsetting (ca. 150 ms), left frontal old/new effect differentiated items accorded correct old vs. correct new responses regardless of whether the items were endorsed as familiar or recollected. This finding extends the range of circumstances under which early, frontally-distributed old/new effects occur, and adds weight to previous suggestions that these effects are a neural correlate of familiarity-driven recognition memory.
Keywords: episodic memory, event-related potential, familiarity, recognition memory, remember-know
1. Introduction
Studies of recognition memory using event-related potentials (ERPs) have consistently reported a ‘left parietal old/new effect’ that differentiates correctly recognized old and new test items. This effect has been interpreted as a neural correlate of recollection, that is, the retrieval of qualitative information about a study episode. By contrast, earlier-onsetting, frontally distributed old/new effects have been interpreted as neural correlates of familiarity-driven recognition (e.g., Curran, 2000; see for reviews, Friedman & Johnson, 2000; Rugg and Allan, 2000).
Although evidence supporting an association between recollection and the left parietal effect is arguably strong, the functional significance of the effect remains uncertain (Wagner et al., 2005). One early suggestion was that it reflects cortical activity supporting the representation of recollected information (Wilding & Rugg, 1996), perhaps reflecting the engagement of what Baddeley (2000) has termed the ‘episodic buffer’. Alternatively, it has been proposed that the effect may index attentional orienting to recollected information (Rugg & Henson, 2002; see also Wagner et al., 2005), rather than the representation or maintenance of such information. To help distinguish between these alternatives, it would be useful to be able to adjudicate between two scenarios that could each account for variation in the magnitude of the effect. On the one hand, the effect might reflect recollection in an all-or-none fashion, such that it is elicited whenever a test item elicits recollection regardless of the amount or content of the information recollected. Alternatively, the effect might be graded, such that its magnitude tracks not just the occurrence of recollection, but the amount of information retrieved. Whereas the ‘representational’ hypothesis of Wilding and Rugg (1996) would be supported by the second of these two scenarios, the former scenario is more compatible with the proposal that the left parietal effect is the neural correlate of an attentional shift or, perhaps, some other control signal that directs processing resources in favor of the recollected information.
Findings from prior studies suggest that the left parietal old/new effect is indeed sensitive to the amount of information recollected, but none of these studies is without interpretational problems. In brief, in all of these studies the amount of information recollected in response to a test item was confounded with variation in the probability that the item actually elicited recollection. Thus, one cannot rule out the possibility that a difference in the magnitude of the parietal old/new effect in the averaged ERP waveforms merely reflects a corresponding difference in the proportion of trials carrying an all-or-none recollection effect. For example, Wilding and Rugg (1996) reported that the parietal old/new effect was greater when elicited by test items accompanied by successful vs. unsuccessful source memory (see also Senkfor & Van Petten, 1998; for review see Friedman & Johnson, 2000). Whereas this finding could be construed as evidence for a graded recollection effect, it is equally compatible with the possibility that items attracting inaccurate source judgments were more likely to have failed to elicit recollection, and to have been recognized as ‘old’ solely on the basis of an acontextual sense of familiarity. Similar difficulties arise in the interpretation of other studies reporting graded parietal old/new effects (e.g., Rugg et al., 1996; Rugg et al., 1995; Wilding, 2000). In each case, it can be argued that the condition giving rise to the smaller effect was the one where accurate recognition judgments were more likely to have resulted from either a ‘lucky guess’ or the influence of familiarity in the absence of recollection.
The current experiment was designed to test the hypothesis that the magnitude of the parietal old/new effect elicited by a retrieval cue reflects the amount of information recollected. A modified remember/know paradigm was used in conjunction with a study task that required an association to be formed between two pictures. On each test trial, subjects were presented with one of the two pictures that had been presented on a study trial, or an unstudied picture. The response options allowed them to signal that they could recollect the picture that had been associated with the test item at study, that they could recollect a detail or details of the study episode other than the associate, that no details could be recollected although the test item was highly familiar or, that the test item was new. The rationale for this procedure rests on the assumption that whereas an R2 judgment depends on retrieval of a sufficiently detailed representation of the study episode to permit recovery of the test item’s pairmate, an R1 response can be made on the basis of only partial retrieval of the encoding episode. We therefore assume that, on average, R2 judgments were associated with recollection of more information than R1 judgments, permitting identification of regions sensitive to amount of information recollected. Thus, by using the two different categories of ‘remember’ response we were able to compare the brain activity associated with recollection of differing amounts of information in the absence of the confounds discussed previously. If the parietal old/new effect is sensitive to the amount of information retrieved, its amplitude will be greater on those trials where recollection of the test item is accompanied by recollection of its studied associate.
2. Results
Two sets of ERP analyses were performed, both of which were restricted to trials associated with correct behavioral responses. The first analysis contrasted ERPs associated with R1, R2, and New responses. The second contrasted ERPs associated with K responses, the combination of R1 and R2 responses, and New responses. These analyses were performed separately because subjects contributing sufficient R1, R2, and New trials (see below) failed to contribute sufficient K trials. Likewise, those subjects with enough K trials lacked sufficient R1 and R2 trials to allow their inclusion in an analysis encompassing all response categories. For this reason, R1 and R2 trials were collapsed to permit an analysis that contrasted K with ‘R’ (that is, R1 and R2 trials combined) and New categories.
2.1. Subject Exclusion Criteria
The principal ERP analyses took the form of contrasts between waveforms elicited by old items accorded R1 and R2 responses, and correctly rejected new items. There was substantial variation across subjects in their distributions of correct recognition decisions to the two Remember categories, with eight subjects showing endorsement rates in one or other category of around 10% or less. These subjects were excluded on the grounds of insufficient trials to form ERPs in one or both conditions. An additional 12 subjects were excluded because, after removal of trials associated with artifact, trial numbers in at least one of the conditions fell below 15.
2.2. Behavioral Performance
The pattern of behavioral performance for the 12 subjects who contributed to the primary ERP analyses did not qualitatively differ from the pattern for the remaining 10 subjects who contributed ERP data to the other analyses reported below.2 Therefore, only the behavioral data from the 12 subjects included in the principal analyses are reported.
2.2.1. Study
Time to signal good image formation at study was calculated separately according to the nature of the subsequent test response. The mean study response times (RTs) for pairs whose test items were later given K, R1, and R2 responses at test were 6005 ms, 6188 ms, and 5937 ms, respectively. A repeated-measures ANOVA revealed a significant effect of response category, F(2, 21.6) = 5.03, p < 0.05. Subsequent pairwise comparisons revealed a significant (p < 0.05) difference between the RTs for items later accorded R1 vs. R2 responses. Study RTs for items later given K vs. R1 and K vs. R2 responses did not differ significantly.
2.2.2. Test
Overall correct recognition rate was 89%, against a false alarm rate of 6%. Table 1 shows the proportion of old and new items attracting each category of response, excluding response omissions. Table 2 shows the mean RTs for correct responses at test. A repeated-measures ANOVA on the K, R1, R2, and New RTs revealed a significant effect, F(2, 22.2) = 25.76, p < 0.001. Pairwise tests indicated that RTs for correctly rejected new items were shorter than those for correctly recognized old items in each response category. In addition, R2 responses were faster than either R1 or K responses (p < 0.01 for all comparisons). An ANOVA on the standard deviations of the RT distributions associated with each response category revealed no significant effect, F(2.1, 22.7) = 1.94.
Table 1.
Percentage of Old and New Items Attracting Each Category of Response
| Item | R2 | R1 | K | New |
|---|---|---|---|---|
| Old | 40.6 | 30.1 | 18.4 | 7.6 |
| New | 0.1 | 0.4 | 5.6 | 91.8 |
Table 2.
Mean Correct Response Latency (ms) by Response Category
| Response | RT (SD) |
|---|---|
| R2 | 1729 (341) |
| R1 | 2168 (386) |
| Know | 2148 (423) |
| New | 1367 (428) |
2.3. R1 vs. R2 vs. New ERPs
As noted above, the principal ERP analyses were restricted to trials associated with correct R1, R2, and New responses. Grand average ERP waveforms from 4 representative scalp locations are shown in Figure 1. Across subjects, the mean number of trials (and range) included in these ERPs were 27 (16–57), 37 (16–71), and 37 (18–54) for R1, R2, and New responses, respectively. Visual inspection of the waveforms reveals multiple points of divergence, including generic old/new effects, as well as effects seemingly specific to one category of old response. Generally, the waveforms for old items are more positive-going than those for new items, and the waveforms for R2 responses are more positive-going than those for R1 responses. The old/new effects appear to onset earlier at anterior than posterior locations. A parietal old/new effect is clearly evident, with a seemingly greater amplitude for R2 than R1 responses. A stimulus-offset response, taking the form of a negative-going deflection peaking around 800 ms, is evident in the frontal waveforms of all conditions.
Figure 1.
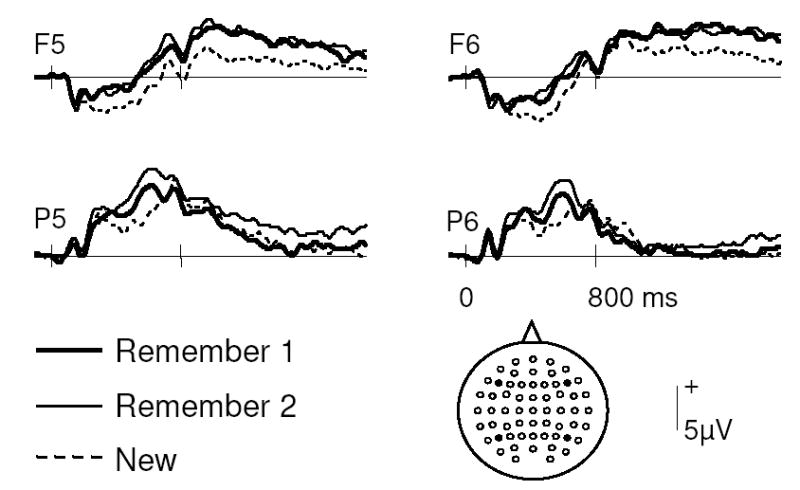
Grand average ERPs to correctly classified new items and to old items categorized as R1 and R2. Waveforms are shown from 4 representative electrode sites as indicated.
Analyses were conducted on the mean ERP amplitudes for the R1, R2, and New response categories over six latency windows: 200–300 ms, 300–500 ms, 500–800 ms, 800–1100 ms, 1100–1400 ms, 1400–1900 ms poststimulus onset. Analyses of the earliest latency window (200–300 ms) were employed to examine the early modulations apparent from visual inspection. The remaining windows are similar to those conventionally used in the analysis of ERP memory retrieval effects (e.g., Duzel et al., 1997). Initial ANOVAs were performed to detect effects due to study status and response category. The electrode locations used in these global ANOVAs are displayed in Figure 2. These electrodes were grouped by hemisphere and further factored into anterior/posterior location. The global ANOVAs employed the factors of response category, hemisphere, anterior/posterior location, and site. Only those effects involving the factor of response category are reported. In the event of a significant effect involving this factor, follow-up pairwise comparisons were performed between response categories using the same factors as those employed in the global ANOVA. Greenhouse-Geisser corrected degrees of freedom were used for all ANOVAs.
Figure 2.
Electrode sites included in the ERP analyses.
Results of the global and subsidiary ANOVAs are summarized in Table 3. ANOVA of the earliest 3 latency regions (200–300 ms, 300–500 ms, and 500–800 ms) gave rise in each case to significant response category effects. No effects were evident, however, for the three later latency regions. For the 200–300 ms region, the global ANOVA revealed a main effect of response category as well as an interaction between response category, anterior/posterior location, and hemisphere. Subsequent comparisons of ERPs elicited by items given R1 and R2 responses revealed a response category by anterior/posterior location by hemisphere interaction. ERPs elicited by items given R2 responses were more positive-going than those given R1 responses over the right hemisphere, F(1,11) = 7.81, p < 0.025, and at posterior scalp sites, F(1,11) = 5.17, p < 0.05. Comparisons of ERPs elicited by correct R1 and New, and R2 and New responses demonstrated that both R1 and R2 ERPs were more positive-going than New ERPs. These early old/new effects were greatest over the left hemisphere and at anterior sites for both the R1 vs. New and R2 vs. New comparisons.
Table 3.
ANOVA Table for the R1 vs. R2 vs. New Analyses
| Contrast | Effect | 200–300 | 300–500 | 500–800 |
|---|---|---|---|---|
| R1/R2/New | RC | F(2.0, 21.8) = 3.56
p < 0.05 |
F(1.4, 15.4) = 8.12
p < 0.01 |
F(1.8, 19.9) = 15.63
p < 0.001 |
| RC/AP/HM | F(1.7, 18.2) = 9.99
p < 0.0025 |
--- | --- | |
| R1/R2 | RC | --- | --- | F(1,11) = 16.68
p < 0.0025 |
| RC/AP/HM | F(1, 11) = 5.62
p < 0.05 |
--- | --- | |
| R1/New | RC | --- | --- | --- |
| RC/HM | --- | --- | F(1,11) = 6.27
p < 0.05 |
|
| RC/AP/HM | F(1, 11) = 14.32
p < 0.005 |
F(1,11) = 6.62
p < 0.05 |
--- | |
| R2/New | RC | F(1,11) = 6.65
p < 0.05 |
F(1,11) = 33.87
p < 0.001 |
F(1,11) = 30.17
p < 0.001 |
| RC/HM | --- | F(1,11) = 6.59
p < 0.05 |
--- | |
| RC/AP/HM | F(1, 11) = 6.70
p < 0.05 |
--- | --- |
Note. Response Category (RC), Anterior/Posterior (AP), Hemisphere (HM).
Old/new effects were also found in the 300–500 ms latency window, where a main effect of response category was revealed by the global ANOVA. Subsidiary ANOVAs comparing R1 vs. New, and R2 vs. New ERPs revealed that R1 and R2 ERPs were each more positive-going than New ERPs, although the response category effect did not quite reach significance for the R1 vs. New comparison, F(1,11) = 4.62, p < 0.06. The ANOVAs also revealed response category by anterior/posterior location by hemisphere and response category by hemisphere interactions for the R1 vs. New and R2 vs. New comparisons, respectively. The R1 old/new effect was greatest at left anterior sites. The R2 old/new effect was maximal over the left hemisphere. The ERPs associated with R1 and R2 responses did not significantly differ during this time interval.
For the 500–800 ms latency region, there was a significant main effect in the global ANOVA. Subsidiary ANOVAs contrasting the response categories with one another revealed that R2 ERPs were more positive-going than R1 and New ERPs (see Figure 1). In addition, the R1 vs. New comparison revealed a response category by hemisphere effect, with R1 ERPs being more positive-going than New ERPs over the left hemisphere.
2.4. Topographic Analyses
The scalp topographies of the differences between R1-New, R2-New, and R2-R1 ERPs are shown in Figure 3 for the three latency regions in which the effects were reliable. To compare their topographies, ANOVAs were performed on the mean amplitude of the R2-R1 and R1-New effects in each latency region, employing the same electrode groupings and site factors as were used in the analyses of effect magnitude. To eliminate the confounding effects of differences in overall amplitude, the data were range-normalized prior to analysis (McCarthy & Wood, 1985). For the 200–300 ms interval, a significant interaction between the two classes of effect, anterior/posterior location, and hemisphere was revealed, F(1,11) = 12.36, p < 0.01. Whereas the R2–R1 difference was maximal over posterior sites, the R1-New difference was maximal over left anterior sites. No significant differences in topography were found for the two classes of effect in the 300–500 ms or 500–800 ms latency regions.
Figure 3.
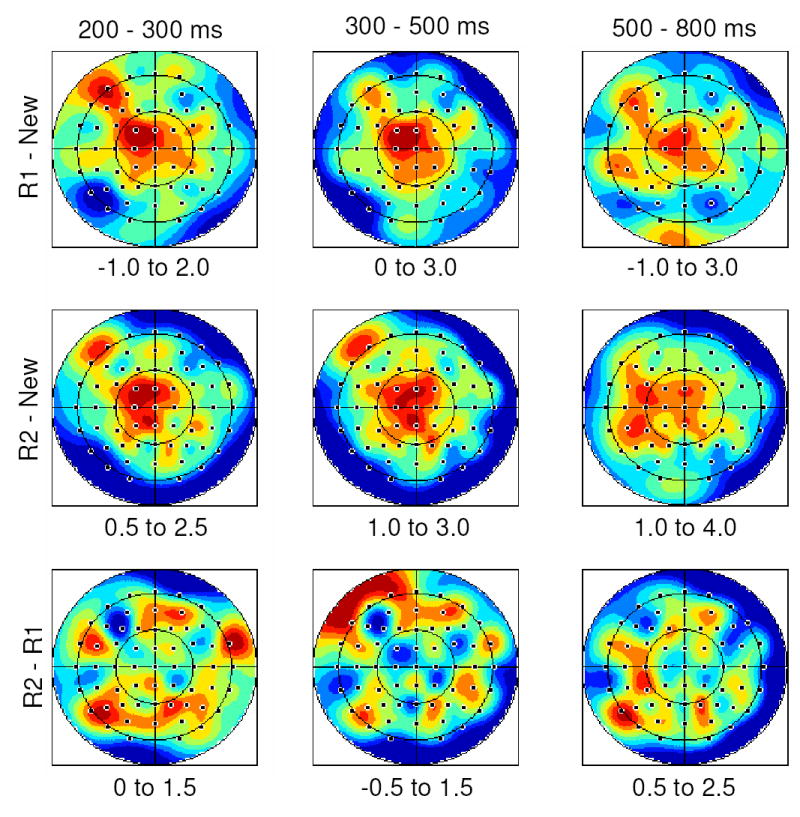
Topographic plots for the 200–300 ms, 300–500 ms, and 500–800 ms intervals for the R1-New (upper), R2-New (middle), and R2-R1 (bottom) subtractions. Maps are scaled by the minima (blue) and maxima (red) of each effect (displayed below in microvolts).
Visual inspection of the topographic plots of the R2-R1 effect across the three latency regions reveals evidence of a left parietal focus in each case (see Figure 3). To formally compare the topography of this effect over time, pairwise ANOVAs were performed to contrast each latency region with the others. The topography of the effect did not significantly differ in any of the contrasts (minimum p > 0.08).
2.5. R2 vs. New ERPs
The combination of low responding in the R1 category and the need to reject artifact-contaminated trials meant that only a minority of the total subject sample contributed data to the foregoing ERP analyses. This raises the concern that the data from subjects who did contribute may not have been representative of that from the entire sample. To address this issue, at least in part, we analyzed the data from an additional group of subjects (n = 10) who contributed at least 15 artifact-free ERP trials to the R2 and New response categories, but for whom there were inadequate numbers of R1 trials. Grand average ERP waveforms from four representative scalp locations are shown for these subjects in Figure 4. Mixed design ANOVAs were employed to contrast these ERPs with the ERPs of subjects who contributed data to all three response categories. On the assumption that the two groups were employing similar strategies and criteria when endorsing items as R2, equivalent patterns of ERP modulation would be expected in each group. This in turn would provide some assurance that the two groups were not employing qualitatively different approaches when deciding whether to endorse items as R2 or R1. For each of the three latency regions analyzed (200–300 ms, 300–500 ms, 500–800 ms), the effects obtained in the original analyses were replicated. Furthermore, no group effects were found for either the 200–300 ms or 300–500 ms latency region. Analysis of the 500–800 ms region however gave rise to a group x response category, F(1, 20) = 8.26, p < 0.05, and a group x response category x site interaction, F(2.7, 54.1) = 3.20, p < 0.05. Inspection of the relevant means suggests that these interactions reflect a tendency for smaller old/new effects, especially near the midline, in the additional subject group. To establish whether this group difference specifically involved the parietal old/new effect, a further ANOVA, restricted to data from posterior sites only, was performed. This gave rise to a significant effect of response category, F(1, 20) = 5.51, p < 0.05, and its interaction with hemisphere, F(1, 20) = 7.82, p < 0.025, but to no evidence of a group effect.
Figure 4.
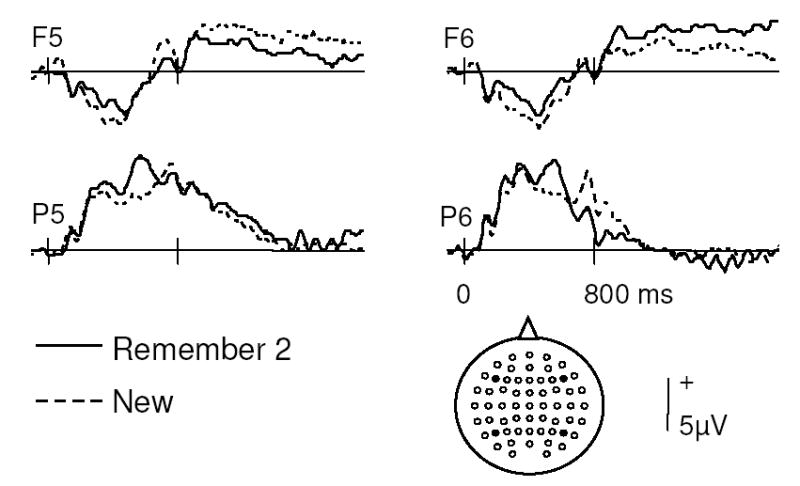
Grand average ERPs to correctly classified new items and to old items categorized as R2. Waveforms are shown from 4 representative electrode sites as indicated.
2.6. K vs. R ERPs
Sixteen subjects contributed sufficient artifact-free trials to permit the formation of ERPs to items given K responses. In order to allow these ERPs to be contrasted with items endorsed as recollected, the ERPs elicited by items endorsed as R1 or R2 were collapsed to form a new category of recollected R items (few of these subjects contributed sufficient trials to allow R1 and R2 ERPs to be analyzed separately). A total of 15 subjects contributed sufficient trials to these ERPs. The mean number of trials (and range) included in each class of waveform were 22 (16–59), 45 (16–95), and 35 (16–54) for correct K, R (that is, R1 + R2), and New responses, respectively. Grand average ERP waveforms at 4 representative scalp locations are shown in Figure 5. ANOVAs were conducted on mean ERP amplitudes with the same electrode groupings as used in the previous analyses. Only those latency regions giving rise to significant effects in the principal analyses were analyzed. In the earliest time interval (200–300 ms), the global ANOVA revealed a response category by anterior/posterior location by site interaction, F(4.6, 64.9) = 3.87, p < 0.01. Subsidiary ANOVAs comparing the R vs. New and K vs. New response categories revealed category by anterior/posterior location by site interactions in each case (F(2.9, 40) = 6.27, p < 0.01 and F(2.5, 35.6) = 3.65, p < 0.05, respectively), indicating that the ERPs associated with items given either an R or a K response were more positive-going than those associated with items given New responses. The ERPs associated with items given K and R responses did not differ significantly in this time interval.
Figure 5.
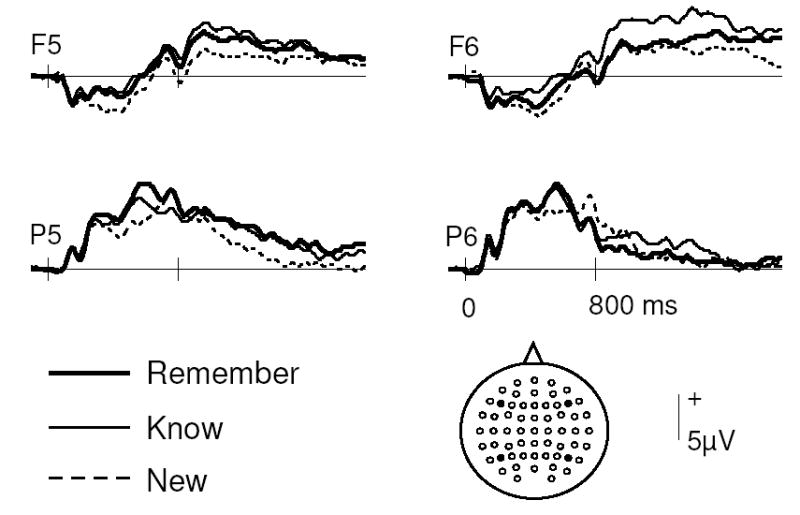
Grand average ERP waveforms from 4 representative scalp locations for items correctly classified with K, R, and New responses.
The global ANOVA of the 300–500 ms data revealed a main effect of response category, F(1.7, 23.3) = 4.00, p < 0.05. Subsidiary comparisons of ERPs associated with items given R and New responses revealed a main effect of response category, F(1, 14) = 11.37, p < 0.01, a response category by hemisphere interaction, F(1, 14)= 5.00, p < 0.05, and a response category by anterior/posterior location by site interaction, F(2.9, 40.7) = 3.75, p < 0.05. These results reflected the fact that the R-New effects were greatest over the left hemisphere and at anterior sites. Subsidiary comparisons of ERPs associated with items given K and New responses revealed a main effect of response category, F(1, 14) = 4.69, p < 0.05, and a response category by anterior/posterior location interaction, F(1, 14) = 4.85, p < 0.05, reflecting the fact that, at anterior sites, ERPs associated with K responses were more positive-going than ERPs to New items. K and R ERPs did not differ significantly in this time interval.
For the 500–800 ms interval, the global ANOVA revealed a response category by hemisphere interaction, F(1.8, 25.6) = 4.07, p < 0.05. Subsidiary comparisons revealed old/new effects for R ERPs by way of a main effect of response category and an interaction with hemisphere, F(1, 14) = 5.20, p < 0.05 and F(1, 14) = 8.71, p < 0.05, respectively. Comparison of left parietal ERPs associated with items endorsed as R and K revealed a main effect of response category, F(1, 14) = 7.08, p < 0.05, the ERPs associated with items given R responses being more positive-going than those for K responses.
3. Discussion
The behavioral results support the assumption that the two categories of ‘remember’ response were associated with retrieval of different kinds of information. RTs to study items later given R2 responses were shorter than those to items later endorsed as R1. This finding lends further support to the validity of the R1/R2 distinction (see Methods): had subjects been selecting between these response options solely on the basis of non-mnemonic information, no relationship with study behavior would have been evident. Rather, it appears that study pairs for which an association was formed relatively easily were more likely to later be fully recollected. Echoing the study data, test RTs were shorter for R2 than for R1 responses. This finding likely reflects a tendency to delay responding if a test item elicited recollection of details other than its associate so as to await the outcome of additional retrieval attempts. This RT difference raises the possibility that the corresponding difference in the amplitude of the left parietal ERP old/new effect is merely a consequence of differential decision or response latencies. This possibility seems unlikely for two reasons. First, if the left parietal old/new effect reflected nothing more than RT differences, correct rejections would have elicited the most positive ERPs in the 500–800ms latency interval of any of the response categories (see Table 2). Second, the across-subjects correlation between the magnitudes of the differences between R2 and R1 RTs, and the mean amplitudes in the 500–800 ms latency region of R2 and R1 left parietal ERPs, was small and far from significant (r =.24, 1–tailed p > 0.2).
As noted previously (see Methods), there are grounds for supposing that on the great majority of trials associated with R2 responses, subjects accurately recollected the pairmate of the test item. The question arises however as to the basis of R1 responses. As noted by a referee, if subjects were prone to make R1 responses even when no study information could be recollected, the larger ERP old/new effects associated with R2 responses would be subject to the same interpretational difficulties that afflict prior studies (see Introduction). That is, the findings may reflect differences between R2 and R1 responses in the probability of an ‘all-or-none’ recollection effect, rather than a true gradation in the amplitude of the effect. Whereas no evidence directly relevant to this issue is available from the present study, evidence was obtained in a companion fMRI study which employed an almost identical procedure to that adopted here (Vilberg and Rugg, submitted). Following the test phase, however, subjects were re-presented with each of the items to which they had made an R1 response and were asked to explain the basis for the response. More than 95% of the responses were reported as being based on retrieval of episodic information (most frequently, details about the study presentation of the test item, such as its location within the display window). There is no reason to suppose that the findings would have been much different in the present study, and hence no reason to think that a significant proportion of R1 responses were associated with a failure of recollection. This conclusion is bolstered by consideration of the proportion of false alarms that were endorsed as R1 and R2 (0.4% and 0.1% respectively, compared with a 5.6% rate for K responses; see Table 1). These very low rates of endorsement (10 subjects made no R1 false alarms at all) suggest that ‘false recollection’ occurred on a negligible proportion of test trials.
Whereas the above discussion suggests that the great majority of R1 and R2 responses were associated with veridical recollection, it leaves open the question whether R2 responses were associated with retrieval of more information than R1 responses. As outlined in the Introduction, we assume that this was the case on the grounds that memory for the pairmate of a test item depends upon recall of information more detailed than that necessary to support an R1 response. Whereas R1 responses might sometimes have been based on recollection of large amounts of episodic detail (albeit excluding the identity of the test item’s pairmate), the task instructions emphasized that recollection of only a single detail about the encoding episode was sufficient to justify such a response. We assume therefore that, on average, R1 responses were associated with less information than R2 responses. We acknowledge however that this assumption is not supported by evidence independent of the findings obtained through the employment of the procedure.
A final concern when interpreting the R2/R1 effects arises from the fact that only a minority of the total subject sample contributed to the relevant analyses. This raises the possibility that the favorable distribution of trials in these subjects was a consequence of adopting criteria for R2 vs. R1 endorsements that is unrepresentative of the population from which they were sampled. For two reasons, this possibility seems unlikely. First, there were no qualitative differences in the pattern of behavioral performance between the subset of subjects who contributed to the R2/R1 analyses and remaining subjects. Second, the comparison of R2 old/new effects between subjects who were and were not excluded from the principal analyses revealed little evidence of qualitative or quantitative differences, especially in relation to the parietal old/new effect.
As in numerous previous ERP studies of recognition memory, waveforms elicited by test items differentiated recollected and new items. R1 ERPs were more positive-going than were new ERPs, and this effect tended to be lateralized to the left parietal scalp (see Figure 3). We assume that this positivity includes a contribution from the generator(s) of the left parietal old/new effect that has been previously linked with successful recollection (see Introduction). Crucially, R2 ERPs elicited an enhanced positivity relative to R1 waveforms, and this difference demonstrated a left lateral parietal maximum. Thus, the difference in the magnitude of both the R2/R1 and R1/New ERP effects likely reflects modulation of the same population of recollection-sensitive neural generators. On the assumption that R2 responses are associated with the retrieval of more episodic information than R1 responses are, these findings support the proposal that the magnitude of the left parietal old/new effect is sensitive to amount of information recollected. As already noted, direct support for this assumption is not available. To the extent that an explanation of the ERP findings in terms of such factors as differential response processing can be discounted, it is difficult however to conceive of an alternative account. Further, the present findings are not alone in suggesting a relation between the magnitude of the left parietal old/new effect and amount of recollected information; rather, they converge with the results of a number of prior studies that employed quite different procedures to segregate test items on the basis of amount recollected (e.g., Rugg et al., 1996; Rugg et al., 1995; Wilding, 2000).
Resolution of the question whether the left parietal old/new effect has an ‘all-or-nothing’ character or, instead, responds in graded fashion according to the amount of retrieved information would contribute significantly to an understanding of the functional significance of the effect. Evidence that the effect was all-or-none would suggest that it reflects a process triggered by the occurrence of recollection, but which does not support or operate on the representation of the recollected content. One example of such a process is the modality-independent, temporoparietally localized ‘circuit-breaker’ that has been proposed to initiate attentional shifts (Astafiev et al., 2006). By contrast, evidence that the left parietal effect is graded is consistent with the view that the effect is a correlate of processes that either support the representation of recollected information, or act on such representations. Examples of the former class of processes would be those that maintain retrieved information in an episodic buffer (Baddeley, 2000), or in working memory more generally. Examples of the latter class are processes that support selection of salient or task-relevant information from among the contents of working memory (Lepsien et al., 2005). As already noted, in conjunction with previous results, the present findings strongly suggest that the left parietal effect is graded. The findings therefore argue against a functional interpretation of the effect as a control or alerting signal, and in favor of some kind of representation-based account.
In addition to the R2/R1 effect evident in the 500–800 ms latency region, an R2/R1 effect was also present in two earlier latency regions (200–300 ms and 300–500 ms). As is evident from Figure 3, these effects also have a focus over the left parietal scalp. Indeed, the contrast of the topography of the earlier R2/R1 effects with the topography of the effect in the 500–800 ms latency interval revealed no significant effect involving latency region. One possibility is that, relative to items attracting R1 responses, R2 items elicited a left parietal effect that was not only greater in magnitude, but also earlier in its onset; that is, fuller recollection was also faster recollection. Thus, in addition to the ‘wait and see’ strategy advanced previously as an explanation for the relatively slow RTs associated with R1 responses, it is also possible that episodic information supporting an R2 response became available more quickly than did the information supporting R1 responses.
As in two other recent ERP studies of recognition memory employing pictorial stimuli (Duarte et al., 2004; Tsivilis et al., 2001), the contrast between hits and correct rejections revealed a remarkably early-onsetting old/new effect (ca. 150 ms) that was topographically distinct from the later-onsetting left parietal effect. The effect was maximal over the frontal scalp, and took the form of greater positivity for correctly identified old items regardless of their assigned response category. The topography of the present effect differs somewhat from the topographies reported previously (Duarte et al., 2004; Tsivilis et al., 2001) in that it demonstrates a more asymmetric and less anterior distribution. The reason for this difference is unclear, and for the present we assume that the prior and current effects share at least some of the same underlying generators and functional correlates. The existence of the effect in the present study extends the circumstances under which it is manifest, and highlights the rapidity with which neural activity can discriminate recently experienced versus novel visual stimuli. The present findings are consistent with those of Duarte et al. (2004) in showing that the effect is insensitive to whether an item elicits recollection. Combined with prior findings that the effect is not elicited by old items that are incorrectly endorsed as new (Duarte et al., 2004; Tsivilis et al., 2001), the present results support the proposal that this early effect is a neural correlate of familiarity-based pictorial recognition memory (Duarte et al., 2004).
3.1. Conclusions
The current study was designed to help adjudicate between two hypotheses about the functional significance of the left parietal ERP old/new effect. Consistent with the hypothesis that the effect indexes the amount of information recollected in response to a test item (Wilding & Rugg, 1996), we found it to be modulated according to the level of episodic detail elicited by a test item.
The question arises as to the intra-cerebral origins of the left parietal effect. On the basis of findings from neuroimaging studies of recognition memory, it has been proposed that the effect is generated in regions of lateral parietal cortex where activity is selectively enhanced by items eliciting recollection (e.g., Wagner et al., 2005; Yonelinas et al., 2005; Wheeler and Buckner, 2004; Rugg et al., 2002). To the extent that these proposals are valid, the present findings lead to the prediction that, like its ERP analogue, the fMRI parietal old/new effect should also be modulated by the amount of information recollected. This prediction has been confirmed in an fMRI study that employed the same study and test tasks as those adopted here (Vilberg and Rugg, submitted).
4. Methods
4.1. Subjects
Subjects were right-handed, native English speakers aged between 18 and 24. A total of 32 individuals (19 female) took part in the experiment (see Results for details of the numbers of subjects contributing to the different analyses, and the rationale for exclusion of subjects). In accordance with the requirements of the UCI Institutional Review Board, which approved this study, all subjects gave informed consent prior to participating.
4.2. Stimuli
Stimuli were color pictures of objects on grey backgrounds. The pool used to create stimulus lists was composed of 403 pictures of objects drawn from the same pool employed by Smith et al. (2004). Allocation of stimuli to experimental conditions was randomized on a subject-specific basis. For each subject, 240 stimuli were randomly selected to be presented at study. An additional 60 pictures were randomly selected to serve as new test stimuli. Thirty pictures were randomly selected from the stimulus pool to be used in a short practice session.
At study, pictures were presented in pairs on a grey background (17 x 22 degrees). The maximum vertical and horizontal visual angle subtended by a stimulus was 3.4 degrees. The members of each stimulus pair were presented in different quadrants of the display monitor. All location combinations occurred with equal probability.1 One hundred twenty of the 240 study pictures were selected to serve as old items at test. Old test items were sampled equally from the four possible study locations. Two buffer trials were added to the beginning of the study list, and three buffer trials were added to the beginning of the test list.
4.3. Procedure
Following the practice session (see below), subjects were fitted with the electrode cap and began the study phase. Study trials consisted of the presentation of two pictures for a maximum of 8 seconds or until a button press was made. After 8 seconds had elapsed or the subject made a response (whichever came sooner), the next study display was immediately presented. A break was given after the 62nd study trial. The study task was to visualize an interaction between the pictures and respond with any key when a “good” visual image had been formed. After completion of the study task, subjects were given a 10 minute break to rest and review the test instructions before beginning the test task. Each test trial consisted of the presentation of a central fixation cross (+) for 690 ms, followed by a centered test picture for 500 ms. After the picture disappeared, the screen was blanked for 120 ms before a question mark was presented. The question mark remained on the screen for 3000 ms and was followed by a blank screen for 560 ms before the next trial commenced. Breaks were given after the 63rd and 123rd test trials.
The test task was a modified Remember/Know procedure in which subjects were required to respond according to the nature of the information retrieved from memory. Four responses were possible: New, Know (K), Remember 1 (R1), and Remember 2 (R2). Instructions on how to use the response categories as well as examples of when to use them were both read by the subjects and verbally explained. Instructions for Know and Remember 1 responses were identical to those used by Gallo, Roediger, and McDermott (2001) with the exception that the instructions were altered to accommodate the employment of pictures. Subjects were instructed to use the Know option when nothing could be recollected about the picture’s study occurrence, but the subject was nonetheless confident that the test picture had been studied. The Remember 1 option was to be used when any aspect of the study episode, however minor, was recollected, other than the identity of the companion picture. The Remember 2 option was to be used only when the identity of the picture that had been paired with the test picture at study could be recalled. Subjects were instructed to use the New response when they judged that a test picture was new or when they were uncertain whether the test picture was old.
Subjects were instructed to use the index, middle, and ring fingers of one hand to make Know, R1, and R2 responses and the index finger of the other hand to respond New. Hand assignment was randomized across subjects. During all experimental tasks, subjects were seated in a dark, sound-attenuated room approximately 1 meter away from a computer screen. A keypad was used to make responses.
Subjects completed a short practice session prior to the application of the electrode cap. This session consisted of one study and one test practice block of 12 and 18 trials, respectively. Subjects received instructions on how to perform each task just prior to its completion. In order to evaluate their comprehension of test instructions, after completing the practice block (see below) subjects were asked to report those items that were paired with test items endorsed as R2. All subjects were later able to accurately report more than 80% of the items. These findings accord well with the results of a preliminary behavioral study that employed a study-test procedure identical to that adopted in the main part of the present study except for the requirement to explicitly recall the pairmate immediately after each R2 response. Across 5 subjects, mean recall accuracy was well over 95%. Together, these findings attest to the validity of the R2 vs. R1 distinction.
4.4. EEG Recording
EEG was recorded from 66 silver/silver chloride active electrodes (BioSemi, Netherlands), 60 of which were arranged in an elasticated cap according to the extended 10–20 system, with the remaining electrodes positioned above and below the left eye, on the outer canthus of each eye, and over each mastoid process. Data were acquired continuously at test at a sampling rate of 256 Hz and an amplifier bandpass of 0 to 67 Hz. EEG was digitally filtered offline with a bandpass of 0.1–19 Hz (3dB points), epoched from 102 ms prestimulus to 1946 ms poststimulus onset, downsampled to 125 Hz, and referenced to linked mastoids. Trials containing artifact resulting from eye movement other than blinks, or from excessive baseline drift were rejected. A regression method was used to correct blink artifacts (see e.g., Henson et al., 2004).
Acknowledgments
KV was supported by NIMH National Research Service Award MH14599-27 and by NSF Graduate Research Fellowship Award D/DGE-0234621. This research was supported by NIMH grant 5R01MH072966.
Footnotes
The purpose of varying the spatial location of the stimuli was to provide additional trial- specific contextual information at encoding which might aid later memory.
Between-subject ANOVAs were performed on the behavioral data of the 12 subjects included in the principal analyses versus the corresponding data from 10 additional subjects included in other ERP analyses. ANOVAs were run on test RTs, study RTs, and the proportions of old and new responses in each response category. No ANOVA revealed any effects involving the factor of group.
Additional analysis of the RT data also casts doubt on the possibility that the ERP differences between R1 and R2 responses are merely a consequence of differential latency jitter. By this account, the smaller left parietal old/new effect for R1 items is a reflection of more variable time-locking of recollective processing than on R2 trials. This possibility is contradicted by the statistically equivalent standard deviations of the RT distributions of R1 and R2 responses (which presumably reflect all sources of inter-trial variance in the chain of cognitive operations linking the presentation of a test item to the resulting response).
References
- Astafiez SV, Shulman GL, Corbetta M. Visuospatial reorienting signals in the human temporo-parietal junction are independent of response selection. European Journal of Neuroscience. 2006;23:591–96. doi: 10.1111/j.1460-9568.2005.04573.x. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends in Cognitive Science. 2000;4:417–23. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Curran T. Brain potentials of recollection and familiarity. Memory & Cognition. 2000;28:923–938. doi: 10.3758/bf03209340. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Winward L, Hayward D, Knight RT. Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Cognitive Brain Research. 2004;18:255–272. doi: 10.1016/j.cogbrainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Duzel E, Yonelinas AP, Mangun GR, Heinze H, Tulving E. Event-related brain potential correlates of two states of conscious awareness in memory. Proceedings of the National Academy of Science: USA. 1997;94:5973–5978. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Johnson R., Jr Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microscopy Research and Techniques. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Roediger HL, McDermott KB. Associate false recognition occurs without strategic criterion shifts. Psychonomic Bulletin & Review. 2001;8:579–586. doi: 10.3758/bf03196194. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rylands A, Ross E, Vuilleumeir P, Rugg MD. The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. Neuroimage. 2004;21:1674–89. doi: 10.1016/j.neuroimage.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Griffin IC, Devlin JT, Nobre AC. Directing spatial attention in mental representations: Interactions between attentional orienting and working-memory load. NeuroImage. 2005;26:733–43. doi: 10.1016/j.neuroimage.2005.02.026. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: An ambiguity associated with analysis of variance models. Electroencephalography and clinical Neurophysiology. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Allan K. Memory retrieval: An electrophysiological perspective. In: Gazzaniga MS, editor. The New Cognitive Neurosciences. 2nd ed. Cambridge, Massachusetts: The MIT Press; 2000. pp. 805–816. [Google Scholar]
- Rugg MD, Cox CJC, Doyle MC, Wells T. Event-related potentials and the recollection of low and high frequency words. Neuropsychologia. 1995;33:471–484. doi: 10.1016/0028-3932(94)00132-9. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA. Episodic memory retrieval: an (event-related) functional neuroimaging perspective. In: Parker AE, Wilding EL, Bussey T, editors. The cognitive neuroscience of memory encoding and retrieval. Hove, UK: Psychology Press; 2002. pp. 83–99. [Google Scholar]
- Rugg MD, Otten LJ, Henson RNA. The neural basis of episodic memory: evidence from functional neuroimaging. Philosophical Transactions of the Royal Society London, B. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Schloerscheidt AM, Doyle MC, Cox CJC, Patching GR. Event-related potentials and the recollection of associative information. Cognitive Brain Research. 1996;4:297–304. doi: 10.1016/s0926-6410(96)00067-5. [DOI] [PubMed] [Google Scholar]
- Senkfor AJ, Van Petten C. Who said what? An event-related potential investigation of source and item memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:1005–1025. doi: 10.1037//0278-7393.24.4.1005. [DOI] [PubMed] [Google Scholar]
- Smith APR, Dolan RJ, Rugg MD. Event-related potential correlates of the retrieval of emotional and nonemotional context. Journal of Cognitive Neuroscience. 2004;16:1–17. doi: 10.1162/089892904970816. [DOI] [PubMed] [Google Scholar]
- Tsivilis D, Otten LJ, Rugg MD. Context effects on the neural correlates of recognition memory: An electrophysiological study. Neuron. 2001;31:497–505. doi: 10.1016/s0896-6273(01)00376-2. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–49. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wilding EL. In what way does the parietal ERP old/new effect index recollection? International Journal of Psychophysiology. 2000;35:81–87. doi: 10.1016/s0167-8760(99)00095-1. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. The Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


