Abstract
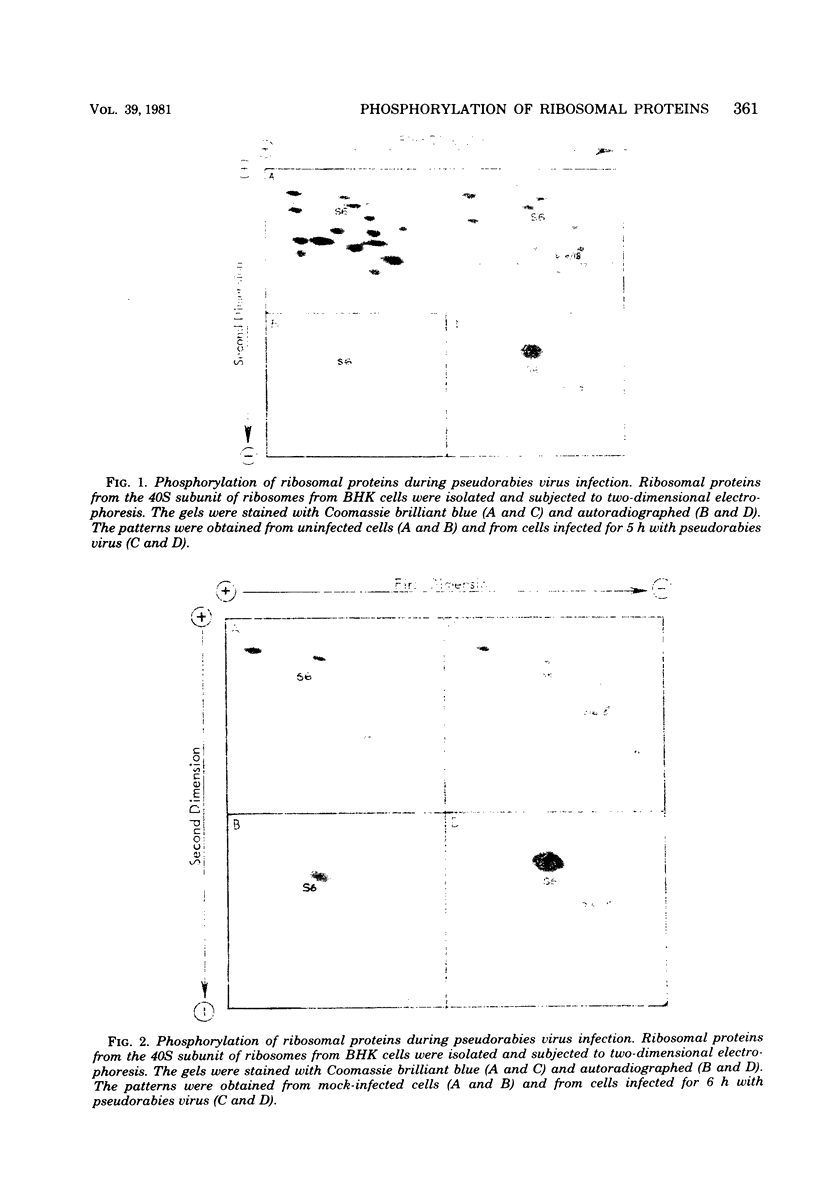
In BHK cells infected with pseudorabies virus, there was a substantial increase in the phosphorylation of ribosomal protein S6. This increase occurred between 2 and 4 h after infection and persisted at least until 9 h. We estimated that in mock-infected cells S6 contained, on an average, one phosphate group per protein chain, whereas in infected cells this rose to between four and five phosphate groups per protein chain. A second ribosomal protein, either S16 or S18, was also phosphorylated after infection. No increase in cyclic AMP was found at the time of phosphorylation. We also found an increased phosphorylation of S6 in herpes simplex virus-infected BHK cells.
Full text
PDF

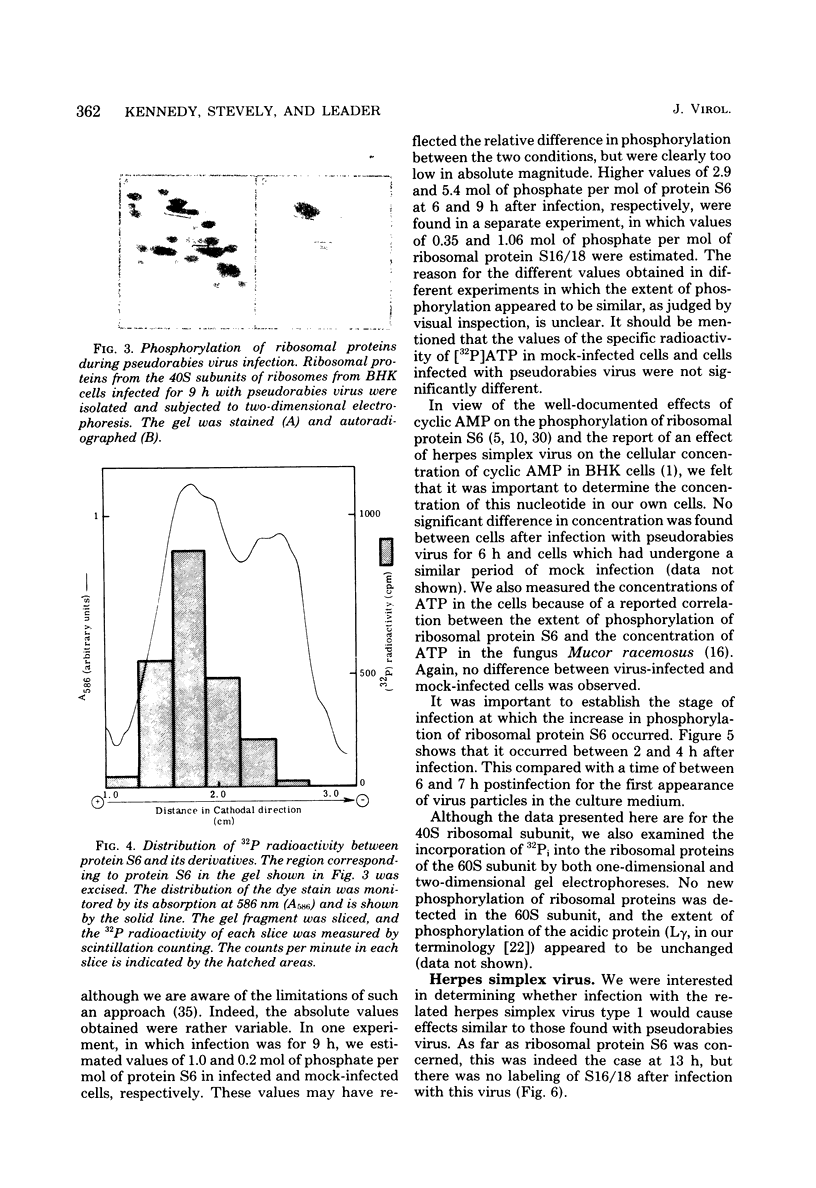
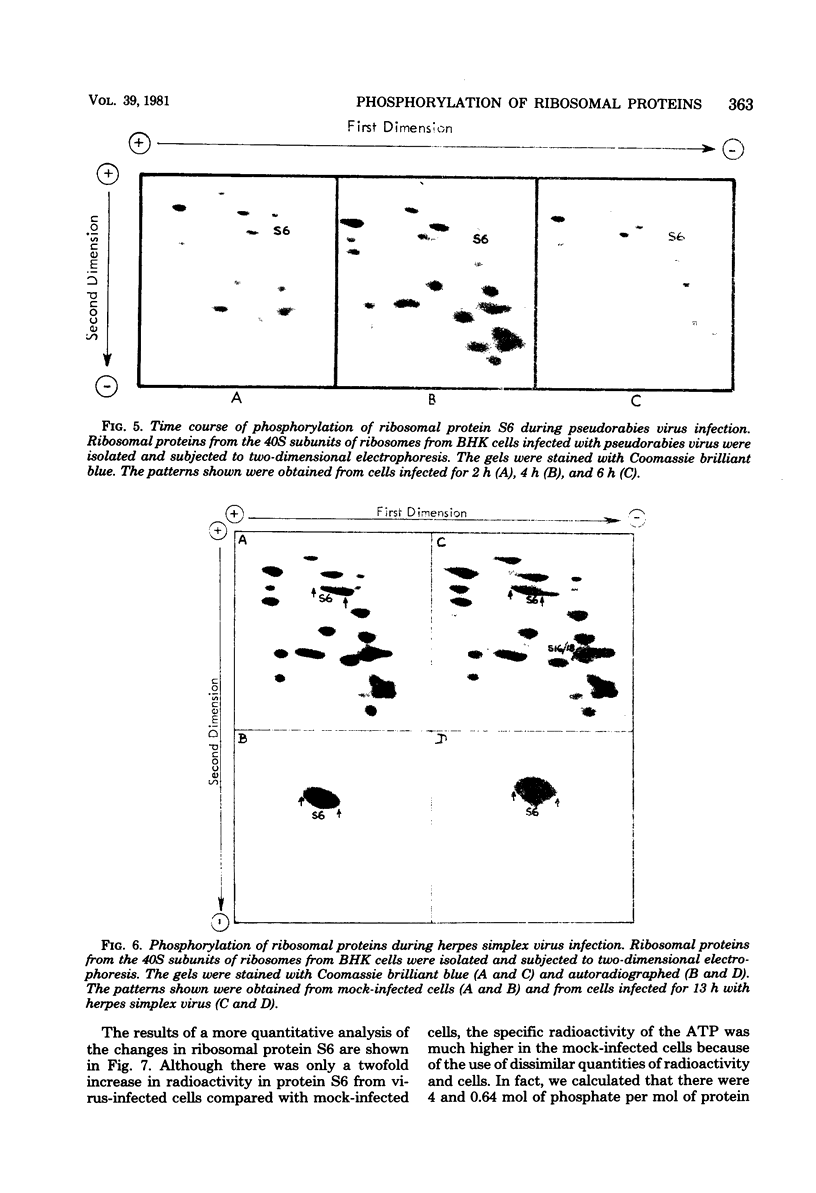
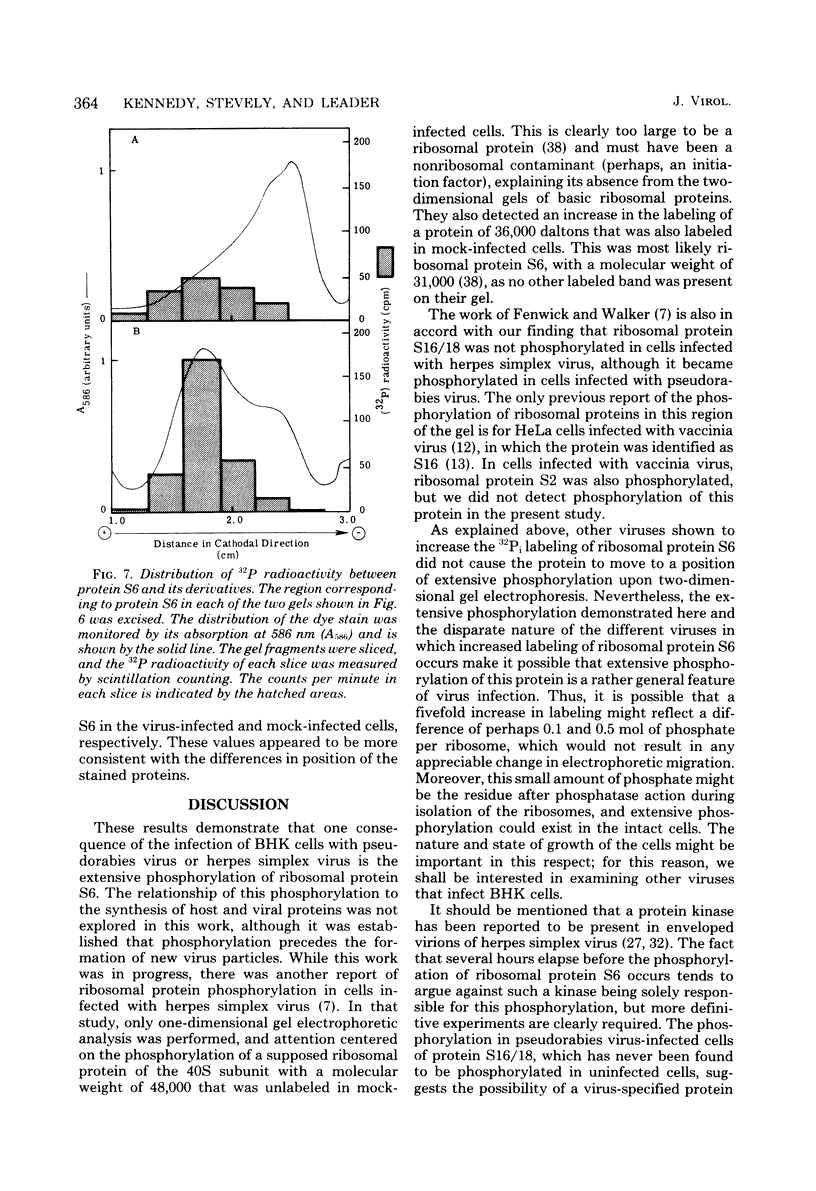


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bittlingmaier K., Schneider D., Falke D. Influence of dibutyryl cyclic AMP on thymidine uptake by herpes simplex virus infected cells and the intracellular level of cyclic AMP. Biochim Biophys Acta. 1977 Aug 2;477(3):228–238. doi: 10.1016/0005-2787(77)90048-x. [DOI] [PubMed] [Google Scholar]
- Blair G. E., Horak I. Phosphorylation of ribosomes in adenovirus infection [proceedings]. Biochem Soc Trans. 1977;5(3):660–661. doi: 10.1042/bst0050660a. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cawthon M. L., Bitte L. F., Krystosek A., Kabat D. Effect of cyclic adenosine 3':5'-monophosphate on ribosomal protein phosphorylation in reticulocytes. J Biol Chem. 1974 Jan 10;249(1):275–278. [PubMed] [Google Scholar]
- Chantler J. K., Stevely W. S. Virus-induced proteins in pseudorabies-infected cells. I. Acid-extractable proteins of the nucleus. J Virol. 1973 Jun;11(6):815–822. doi: 10.1128/jvi.11.6.815-822.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collatz E., Wool I. G., Lin A., Stöffler G. The isolation of eukaryotic ribosomal proteins. The purification and characterization of the 40 S ribosomal subunit proteins S2, S3, S4, S5, S6, S7, S8, S9, S13, S23/S24, S27, and S28. J Biol Chem. 1976 Aug 10;251(15):4666–4672. [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J. Phosphorylation of a ribosomal protein and of virus-specific proteins in cells infected with herpes simplex virus. J Gen Virol. 1979 Nov;45(2):397–405. doi: 10.1099/0022-1317-45-2-397. [DOI] [PubMed] [Google Scholar]
- Gressner A. M., Wool I. G. Influence of glucagon and cyclic adenosine 3':5'-monophosphate on the phosphorylation of rat liver ribosomal protein S6. J Biol Chem. 1976 Mar 10;251(5):1500–1504. [PubMed] [Google Scholar]
- Gressner A. M., Wool I. G. The phosphorylation of liver ribosomal proteins in vivo. Evidence that only a single small subunit protein (S6) is phosphorylated. J Biol Chem. 1974 Nov 10;249(21):6917–6925. [PubMed] [Google Scholar]
- Gressner A. M., Wool I. G. The stimulation of the phosphorylation of ribosomal protein S6 by cycloheximide and puromycin. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1482–1490. doi: 10.1016/0006-291x(74)90365-9. [DOI] [PubMed] [Google Scholar]
- KAPLAN A. S., VATTER A. E. A comparison of herpes simplex and pseudorabies viruses. Virology. 1959 Apr;7(4):394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. Characterization and regulatory aspects. Biochemistry. 1970 Oct 13;9(21):4160–4175. doi: 10.1021/bi00823a019. [DOI] [PubMed] [Google Scholar]
- Kaerlein M., Horak I. Identification and characterization of ribosomal proteins phosphorylated in vaccinia-virus-infected HeLa cells. Eur J Biochem. 1978 Oct 16;90(3):463–469. doi: 10.1111/j.1432-1033.1978.tb12625.x. [DOI] [PubMed] [Google Scholar]
- Kaerlein M., Horak I. Phosphorylation of ribosomal proteins in HeLa cells infected with vaccinia virus. Nature. 1976 Jan 15;259(5539):150–151. doi: 10.1038/259150a0. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Larsen A., Sypherd P. S. Physiological control of phosphorylation ribosomal protein S6 in Mucor racemosus. J Bacteriol. 1980 Jan;141(1):20–25. doi: 10.1128/jb.141.1.20-25.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastick S. M., McConkey E. H. Exchange and stability of HeLa ribosomal proteins in vivo. J Biol Chem. 1976 May 25;251(10):2867–2875. [PubMed] [Google Scholar]
- Lastick S. M., Nielsen P. J., McConkey E. H. Phosphorylation of ribosomal protein S6 in suspension cultured HeLa cells. Mol Gen Genet. 1977 Apr 29;152(3):223–230. doi: 10.1007/BF00693074. [DOI] [PubMed] [Google Scholar]
- Leader D. P. A tracking marker for the first dimension of the two-dimensional gel electrophoresis of ribosomal proteins. J Biochem Biophys Methods. 1980 Oct;3(4):247–248. doi: 10.1016/0165-022x(80)90064-0. [DOI] [PubMed] [Google Scholar]
- Leader D. P., Coia A. A., Fahmy L. H. The extent of phosphorylation of the acidic 60S ribosomal phosphoprotein, Lgamma, in Krebs II ascites cells and in the skeletal muscle of normal and diabetic rats. Biochem Biophys Res Commun. 1978 Jul 14;83(1):50–58. doi: 10.1016/0006-291x(78)90396-0. [DOI] [PubMed] [Google Scholar]
- Leader D. P., Coia A. A. The phosphorylation of an acidic protein of the large ribosomal subunit of Krebs II ascites cells. Biochem J. 1977 Jan 15;162(1):199–200. doi: 10.1042/bj1620199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader D. P., Coia A. A. The phosphorylation of ribosomal protein S6 on the monoribosomes and polyribosomes of baby hamster kidney fibroblasts. FEBS Lett. 1978 Jun 15;90(2):270–274. doi: 10.1016/0014-5793(78)80383-4. [DOI] [PubMed] [Google Scholar]
- Leader D. P., Coia A. A. The phosphorylation of ribosomal proteins L14 and S3 in Krebs II ascites cells. Biochim Biophys Acta. 1978 Jun 22;519(1):224–223. doi: 10.1016/0005-2787(78)90075-8. [DOI] [PubMed] [Google Scholar]
- Leader D. P. Enzymic iodination of eukaryotic ribosomal subunits. Characterization and analysis by two-dimensional gel electrophoresis. Biochem J. 1975 Nov;152(2):373–378. doi: 10.1042/bj1520373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader D. P., Rankine A. D., Coia A. A. The phosphorylation of ribosomal protein S6 in baby hamster kidney fibroblasts. Biochem Biophys Res Commun. 1976 Aug 23;71(4):966–974. doi: 10.1016/0006-291x(76)90749-x. [DOI] [PubMed] [Google Scholar]
- Lemaster S., Roizman B. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of the polypeptides phosphorylated in the virion. J Virol. 1980 Sep;35(3):798–811. doi: 10.1128/jvi.35.3.798-811.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini O. H., Kruppa J. Ribosomal phosphoproteins of mouse myeloma cells. Changes in the degree of phosphorylation induced by hypertonic initiation block. Eur J Biochem. 1979 Apr 2;95(2):349–358. doi: 10.1111/j.1432-1033.1979.tb12972.x. [DOI] [PubMed] [Google Scholar]
- Rankine A. D., Leader D. P., Coia A. A. The phosphorylation of the ribosomal proteins of Krebs II ascites cells. Biochim Biophys Acta. 1977 Jan 20;474(2):293–307. doi: 10.1016/0005-2787(77)90203-9. [DOI] [PubMed] [Google Scholar]
- Roberts S., Ashby D. Ribosomal protein phosphorylation in rat cerebral cortex in vitro. Influence of cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1978 Jan 10;253(1):288–296. [PubMed] [Google Scholar]
- Rubenstein A. S., Gravell M., Darlington R. Protein kinase in enveloped herpes simplex virions. Virology. 1972 Oct;50(1):287–290. doi: 10.1016/0042-6822(72)90374-1. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Blair G. E. Polypeptide phosphorylation in adenovirus-infected cells. J Gen Virol. 1977 Jan;34(1):19–35. doi: 10.1099/0022-1317-34-1-19. [DOI] [PubMed] [Google Scholar]
- Stevely W. S. Virus-induced proteins in pseudorabies-infected cells. II. Proteins of the virion and nucleocapsid. J Virol. 1975 Oct;16(4):944–950. doi: 10.1128/jvi.16.4.944-950.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Siegmann M., Gordon J. Multiple phosphorylation of ribosomal protein S6 during transition of quiescent 3T3 cells into early G1, and cellular compartmentalization of the phosphate donor. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3952–3956. doi: 10.1073/pnas.76.8.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Siegmann M., Kubler A. M., Gordon J., Jimenez de Asua L. Regulation of 40S ribosomal protein S6 phosphorylation in Swiss mouse 3T3 cells. Cell. 1980 Apr;19(4):1015–1023. doi: 10.1016/0092-8674(80)90092-6. [DOI] [PubMed] [Google Scholar]
- Treloar M. A., Treloar M. E., Kisilevsky R. Ethionine and the phosphorylation of ribosomal protein S6. J Biol Chem. 1977 Sep 10;252(17):6217–6221. [PubMed] [Google Scholar]
- Wool I. G. The structure and function of eukaryotic ribosomes. Annu Rev Biochem. 1979;48:719–754. doi: 10.1146/annurev.bi.48.070179.003443. [DOI] [PubMed] [Google Scholar]
- Zinker S., Warner J. R. The ribosomal proteins of Saccharomyces cerevisiae. Phosphorylated and exchangeable proteins. J Biol Chem. 1976 Mar 25;251(6):1799–1807. [PubMed] [Google Scholar]