Abstract
Temperature-sensitive (ts) mutant tsD1 of vesicular stomatitis virus, New Jersey serotype, is the sole representative of complementation group D. Clones derived from this mutant exhibited three different phenotypes with respect to electrophoretic mobility of the G and N polypeptides of the virion in sodium dodecyl sulfate-polyacrylamide gel. Analysis of non-ts pseudorevertants showed that none of the three phenotypes was associated with the temperature sensitivity of mutant tsD1. Additional phenotypes, some also involving the NS polypeptide, appeared during sequential cloning, indicating that mutations were generated at high frequency during replication of tsD1. Furthermore, mutations altering the electrophoretic mobility of the G, N, NS, and M polypeptides were induced in heterologous viruses multiplying in the same cells as tsD1. These heterologous viruses included another complementing ts mutant of vesicular stomatitis virus New Jersey and ts mutants of vesicular stomatitis virus Indiana and Chandipura virus. Complete or incomplete virions of tsD1 appeared to be equally efficient inducers of mutations in heterologous viruses. Analysis of the progeny of a mixed infection of two complementing ts mutants of vesicular stomatitis virus New Jersey with electrophoretically distinguishable G, N, NS, and M proteins yielded no recombinants and excluded recombination as a factor in the generation of the electrophoretic mobility variants. In vitro translation of total cytoplasmic RNA from BHK cells indicated that post-translational processing was not responsible for the aberrant electrophoretic mobility of the N, NS, and M protein mutants. Aberrant glycosylation could account for three of four G protein mutants, however. Some clones of tsD1 had an N polypeptide which migrated faster in sodium dodecyl sulfate-polyacrylamide gel than did the wild type, suggesting that the polypeptide might be shorter by about 10 amino acids. Determination of the nucleotide sequence to about 200 residues from each terminus of the N gene of one of these clones, a revertant, and the wild-type parent revealed no changes compatible with synthesis of a shorter polypeptide by premature termination or late initiation of translation. The sequence data indicated, however, that the N-protein mutant and its revertant differed from the parental wild type in two of the 399 nucleotides determined. These sequencing results and the phenomenon of enhanced mutability associated with mutant tsD1 reveal that rapid and extensive evolution of the viral genome can occur during the course of normal cytolytic infection of cultured cells.
Full text
PDF









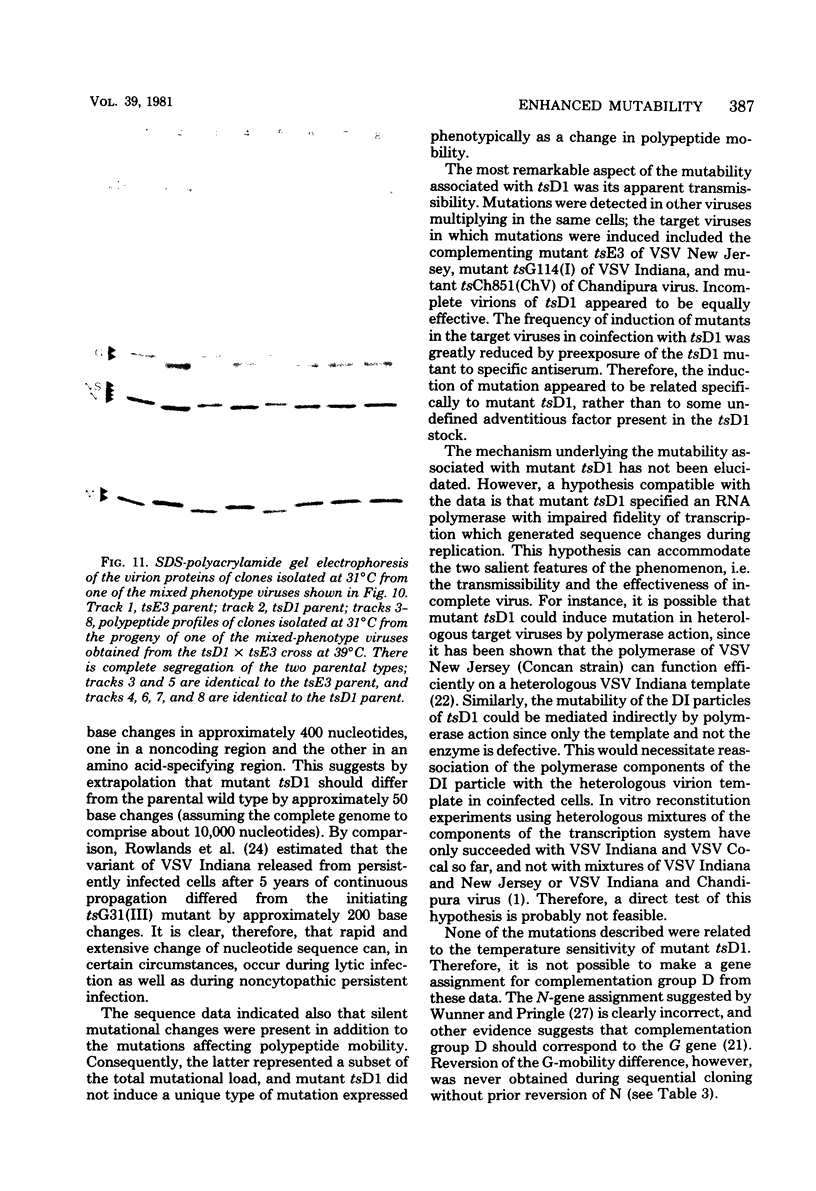


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Emerson S. U., Flamand A. Reconstitution of infectivity and transcriptase activity of homologous and heterologous viruses: vesicular stomatitis (Indiana serotype), Chandipura, vesicular stomatitis (New Jersey serotype), and Cocal viruses. J Virol. 1974 Jul;14(1):139–144. doi: 10.1128/jvi.14.1.139-144.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J. P., Bishop D. H., Kang C. Y., Coffin J., Schnitzlein W. M., Reichmann M. E., Shope R. E. Oligonucleotide fingerprints of RNA species obtained from rhabdoviruses belonging to the vesicular stomatitis virus subgroup. J Virol. 1977 Jul;23(1):152–166. doi: 10.1128/jvi.23.1.152-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972 Aug;10(2):297–309. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D., Pringle C. R., Szilágyi J. F. Temperature-sensitive mutants of complementation group E of vesicular stomatitis virus New Jersey serotype possess altered NS polypeptides. J Virol. 1979 Aug;31(2):325–333. doi: 10.1128/jvi.31.2.325-333.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadkari D. A., Pringle C. R. Temperature-sensitive mutants of Chandipura virus. I. Inter- and intragroup complementation. J Virol. 1980 Jan;33(1):100–106. doi: 10.1128/jvi.33.1.100-106.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadkari D. A., Pringle C. R. Temperature-sensitive mutants of Chandipura virus. II. Phenotypic characteristics of the six complementation groups. J Virol. 1980 Jan;33(1):107–114. doi: 10.1128/jvi.33.1.107-114.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Grabau E. A., Jones C. L., Semler B. L. Evolution of multiple genome mutations during long-term persistent infection by vesicular stomatitis virus. Cell. 1979 Mar;16(3):495–504. doi: 10.1016/0092-8674(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Crombie I. K., Subak-Sharpe J. H. Control of protein synthesis in herpesvirus-infected cells: analysis of the polypeptides induced by wild type and sixteen temperature-sensitive mutants of HSV strain 17. J Gen Virol. 1976 Jun;31(3):347–372. doi: 10.1099/0022-1317-31-3-347. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Pringle C. R. Comparisons of nucleotide sequences in the genomes of the New Jersey and Indiana serotypes of vesicular stomatitis virus. J Virol. 1980 Jan;33(1):69–77. doi: 10.1128/jvi.33.1.69-77.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A. Sequence of 200 nucleotides at the 3'-terminus of the genome RNA of vesicular stomatitis virus. Nucleic Acids Res. 1979 Jul 25;6(10):3199–3211. doi: 10.1093/nar/6.10.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Turnbull N. T. Analysis of the 3'-terminal nucleotide sequence of vesicular stomatitis virus N protein mRNA. Nucleic Acids Res. 1978 Nov;5(11):4007–4024. doi: 10.1093/nar/5.11.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A., Leavitt R. W., Kingsbury D. T., Holland J. J. Natural selection of mutants of vesicular stomatitis virus by cultured cells of Drosophila melanogaster. J Gen Virol. 1973 Sep;20(3):341–351. doi: 10.1099/0022-1317-20-3-341. [DOI] [PubMed] [Google Scholar]
- Noel D., Nikaido K., Ames G. F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. doi: 10.1021/bi00586a017. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M. The cell-free synthesis of herpesvirus-induced polypeptides. Virology. 1977 May 1;78(1):349–353. doi: 10.1016/0042-6822(77)90109-x. [DOI] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B., Stevenson M. Isolation and characterization of temperature-sensitive mutants of vesicular stomatitis virus, New Jersey serotype. J Virol. 1971 Dec;8(6):836–841. doi: 10.1128/jvi.8.6.836-841.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repik P., Flamand A., Bishop D. H. Synthesis of RNA by mutants of vesicular stomatitis virus (Indiana serotype) and the ability of wild-type VSV New Jersey to complement the VSV Indiana ts G I-114 transcription defect. J Virol. 1976 Oct;20(1):157–169. doi: 10.1128/jvi.20.1.157-169.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Katz F. N., Lodish H. F. Glycosylation of a membrane protein is restricted to the growing polypeptide chain but is not necessary for insertion as a transmembrane protein. Cell. 1978 Dec;15(4):1447–1454. doi: 10.1016/0092-8674(78)90068-5. [DOI] [PubMed] [Google Scholar]
- Rowlands K., Grabau E., Spindler K., Jones C., Semler B., Holland J. Virus protein changes and RNA termini alterations evolving during persistent infection. Cell. 1980 Apr;19(4):871–880. doi: 10.1016/0092-8674(80)90078-1. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Holland J. J. Persistent vesicular stomatitis virus infection mediates base substitutions in viral RNA termini. J Virol. 1979 Nov;32(2):420–428. doi: 10.1128/jvi.32.2.420-428.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Kaesberg P. Acrylamide gel electrophoresis of bacteriophage Q beta: electrophoresis of the intact virions and of the viral proteins. Virology. 1970 Oct;42(2):437–452. doi: 10.1016/0042-6822(70)90287-4. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. A temperature-sensitive mutant of vesicular stomatitis virus with two abnormal virus proteins. J Gen Virol. 1974 Apr;23(1):97–106. doi: 10.1099/0022-1317-23-1-97. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. Protein synthesis in BHK21 cells infected with vesicular stomatitis virus. II. ts Mutants of the New Jersey serotype. Virology. 1972 Oct;50(1):250–253. doi: 10.1016/0042-6822(72)90365-0. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Zweers A., Cohen L. H. Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes. Biochem Biophys Res Commun. 1978 May 30;82(2):532–539. doi: 10.1016/0006-291x(78)90907-5. [DOI] [PubMed] [Google Scholar]













