Abstract
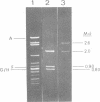
HindIII restriction endonuclease fragments of DNA from temperate Bacillus subtilis bacteriophage SP02 were cloned in B. subtilis by using the plasmid pC194. Three hybrid plasmids which permit growth of the mutant SP02 susL244 in suppressor-negative bacteria were isolated. SP02 gene L is thought to code for a DNA polymerase essential for autonomous replication of SP02 DNA. Extracts of bacteria carrying one of these hybrid plasmids, pC194-96, had 10- to 30-fold increased DNA polymerase activity. The plasmid-induced DNA polymerase activity differed from that of the known B. subtilis DNA polymerases in several respects. The results of the experiments support the idea that phage SP02 codes for a new DNA polymerase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arwert F., Rutberg L. Induction of prophage SPO2 in Bacillus subtilis by 6-(para)-hydroxyphenylazouracil. J Virol. 1974 Dec;14(6):1470–1475. doi: 10.1128/jvi.14.6.1470-1475.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice L., Eiserling F. A., Romig W. R. Structure of bacillus subtilis phage SPO2 and its DNA: similarity of Bacillus subtilis phages SPO2, phi 1O5 and SPP1. Biochem Biophys Res Commun. 1969 Feb 21;34(4):398–403. doi: 10.1016/0006-291x(69)90395-7. [DOI] [PubMed] [Google Scholar]
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canosi U., Morelli G., Trautner T. A. The relationship between molecular structure and transformation efficiency of some S. aureus plasmids isolated from B. subtilis. Mol Gen Genet. 1978 Nov 9;166(3):259–267. doi: 10.1007/BF00267617. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Ehrlich S. D. Replication and expression of plasmids from Staphylococcus aureus in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1680–1682. doi: 10.1073/pnas.74.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass K. B., Cozzarelli N. R. Further genetic and enzymological characterization of the three Bacillus subtilis deoxyribonucleic acid polymerases. J Biol Chem. 1973 Nov 25;248(22):7688–7700. [PubMed] [Google Scholar]
- Gass K. B., Hill T. C., Goulian M., Strauss B. S., Cozzarelli N. R. Altered deoxyribonucleic acid polymerase activity in a methyl methanesulfonate-sensitive mutant of Bacillus subtilis. J Bacteriol. 1971 Oct;108(1):364–374. doi: 10.1128/jb.108.1.364-374.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordănescu S. Recombinant plasmid obtained from two different, compatible staphylococcal plasmids. J Bacteriol. 1975 Nov;124(2):597–601. doi: 10.1128/jb.124.2.597-601.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keggins K. M., Lovett P. S., Duvall E. J. Molecular cloning of genetically active fragments of Bacillus DNA in Bacillus subtilis and properties of the vector plasmid pUB110. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1423–1427. doi: 10.1073/pnas.75.3.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft J., Bernhard K., Goebel W. Recombinant plasmids capable to replication in B. subtilis and E. coli. Mol Gen Genet. 1978 Jun 1;162(1):59–67. doi: 10.1007/BF00333851. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Löfdahl S., Sjöström J. E., Philipson L. Characterization of small plasmids from Staphylococcus aureus. Gene. 1978 Apr;3(2):145–159. [PubMed] [Google Scholar]
- Montenegro M. A., Esche H., Trautner T. A. Induction of mutations in B. subtilis phage SPP1 by growth on host cells carrying a mutator DNA polymerase III. Mol Gen Genet. 1976 Dec 8;149(2):131–134. doi: 10.1007/BF00332880. [DOI] [PubMed] [Google Scholar]
- OKAZAKI T., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XV. PURIFICATION AND PROPERTIES OF A POLYMERASE FROM BACILLUS SUBTILIS. J Biol Chem. 1964 Jan;239:259–268. [PubMed] [Google Scholar]
- Rima B. K., Takahashi I. Deoxyribonucleoside-requiring mutants of Bacillus subtilis. J Gen Microbiol. 1978 Jul;107(1):139–145. doi: 10.1099/00221287-107-1-139. [DOI] [PubMed] [Google Scholar]
- Rutberg L., Armentrout R. W. Deoxyribonucleic acid polymerase activity in a deoxyribonucleic acid polymerase I-deficient mutant of Bacillus subtilis infected with temperature bacteriophage SPO2. J Virol. 1972 Oct;10(4):658–660. doi: 10.1128/jvi.10.4.658-660.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg L., Armentrout R. W., Jonasson J. Unrelatedness of temperate Bacillus subtilis bacteriophages SP02 and phi105. J Virol. 1972 May;9(5):732–737. doi: 10.1128/jvi.9.5.732-737.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Kawano N. Cloning vehicles for the homologous Bacillus subtilis host-vector system. Gene. 1980 Jul;10(2):131–136. doi: 10.1016/0378-1119(80)90130-4. [DOI] [PubMed] [Google Scholar]
- Yasunaka K., Tsukamoto H., Okubo S., Horiuchi T. Isolation and properties of suppressor-sensitive mutants of Bacillus subtilis bacteriophage SP02. J Virol. 1970 Jun;5(6):819–821. doi: 10.1128/jvi.5.6.819-821.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda Y., Graham S., Young F. E. Restriction-fragment map of the temperate Bacillus subtilis bacteriophage SPO2. Gene. 1979 Sep;7(1):51–68. doi: 10.1016/0378-1119(79)90042-8. [DOI] [PubMed] [Google Scholar]