Abstract
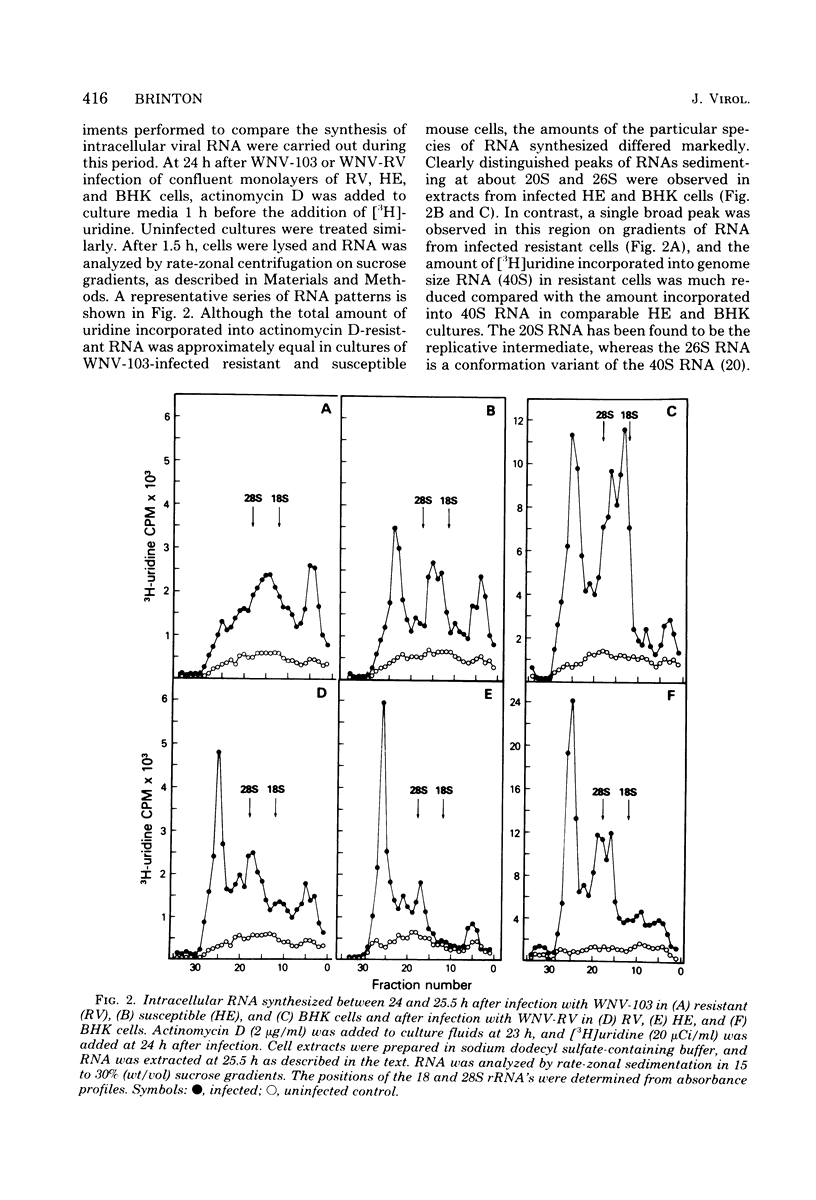
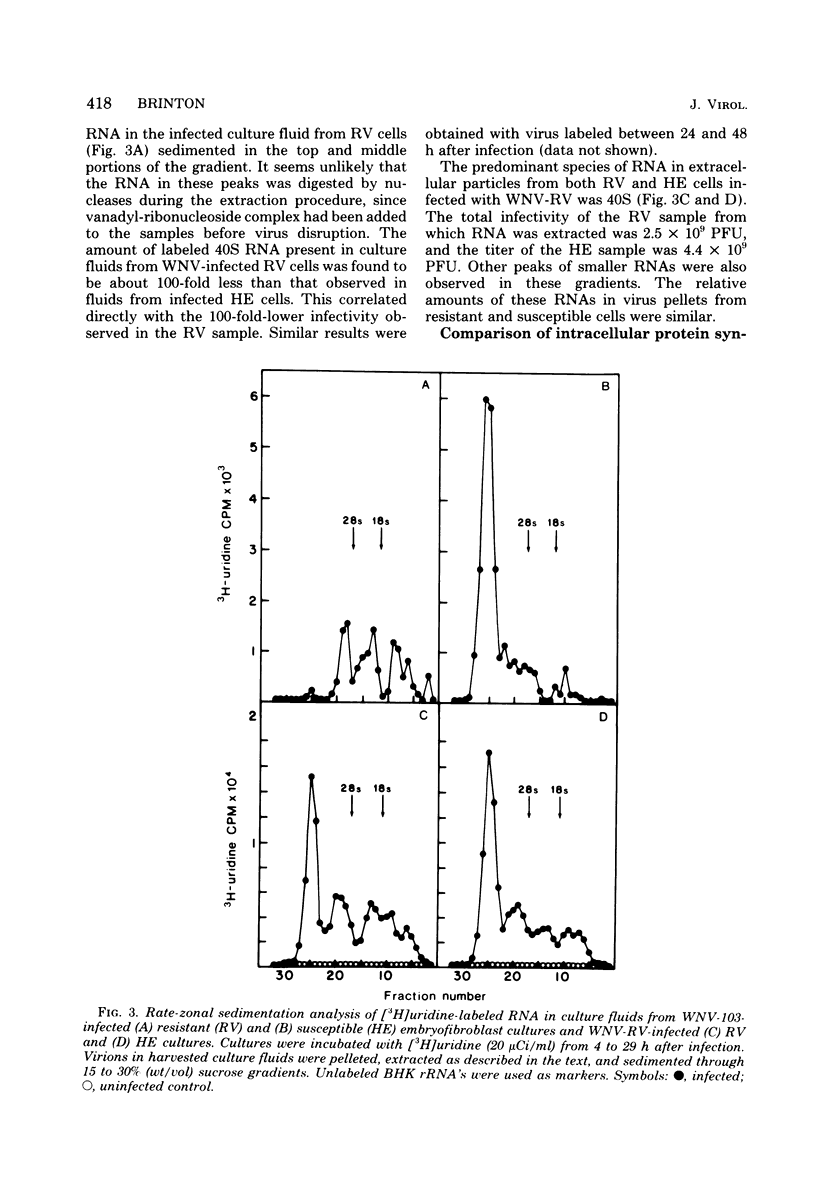
Flavivirus-resistant mouse embryofibroblasts (C3H/RV) that were infected with West Nile virus, strain E101 (WNV), yielded fewer infectious virions than did cultures of congenic susceptible cells (C3H/HE). Analysis of intracellular viral RNA synthesis indicated that the incorporation of [3H]uridine into 40S genome RNA was markedly reduced in resistant cells, and about 100-fold less labeled 40S RNA was found in pelleted extracellular virions from resistant cultures than in those from susceptible ones. A non-temperature-sensitive mutant of WNV isolated from culture fluid of a persistently infected culture of genetically resistant mouse cells was found to produce higher yields of infectious virus than the parental WNV used to initiate the persistent infection. Analysis of intracellular actinomycin D-resistant RNA indicated that the mutant virus (WNV-RV) was more efficient at incorporating [3H]uridine into 40S RNA in resistant cells than was the parental virus. WNV-RV also synthesized 40S RNA more efficiently than parental virus in congenic susceptible cells and in BHK cells. Analysis of the incorporation of [35S]methionine into viral proteins was likewise enhanced in WNV-RV-infected cells. The WNV-RV mutant provides a tool for studying the regulation of transcription of flavivirus RNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinton M. A. Genetically controlled resistance to flavivirus and lactate-dehydrogenase-elevating virus-induced disease. Curr Top Microbiol Immunol. 1981;92:1–14. doi: 10.1007/978-3-642-68069-4_1. [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Kennedy S. I. Purification and polypeptide composition of Semliki Forest virus RNA polymerase. J Gen Virol. 1976 Sep;32(3):395–411. doi: 10.1099/0022-1317-32-3-395. [DOI] [PubMed] [Google Scholar]
- Darnell M. B., Koprowski H. Genetically determined resistance to infection with group B arboviruses. II. Increased production of interfering particles in cell cultures from resistant mice. J Infect Dis. 1974 Mar;129(3):248–256. doi: 10.1093/infdis/129.3.248. [DOI] [PubMed] [Google Scholar]
- Darnell M. B., Koprowski H., Lagerspetz K. Genetically determined resistance to infection with group B arboviruses. I. Distribution of the resistance gene among various mouse populations and characteristics of gene expression in vivo. J Infect Dis. 1974 Mar;129(3):240–247. doi: 10.1093/infdis/129.3.240. [DOI] [PubMed] [Google Scholar]
- GOODMAN G. T., KOPROWSKI H. Study of the mechanism of innate resistance to virus infection. J Cell Comp Physiol. 1962 Jun;59:333–373. doi: 10.1002/jcp.1030590313. [DOI] [PubMed] [Google Scholar]
- Haller O., Arnheiter H., Gresser I., Lindenmann J. Genetically determined, interferon-dependent resistance to influenza virus in mice. J Exp Med. 1979 Mar 1;149(3):601–612. doi: 10.1084/jem.149.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lynch C. J., Hughes T. P. The Inheritance of Susceptibility to Yellow Fever Encephalitis in Mice. Genetics. 1936 Mar;21(2):104–112. doi: 10.1093/genetics/21.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki M., Käriäinen L. Solubilized RNA replication complex from Semliki Forest virus-infected cells. Virology. 1979 Oct 30;98(2):298–307. doi: 10.1016/0042-6822(79)90553-1. [DOI] [PubMed] [Google Scholar]
- Sabin A. B. Nature of Inherited Resistance to Viruses Affecting the Nervous System. Proc Natl Acad Sci U S A. 1952 Jun;38(6):540–546. doi: 10.1073/pnas.38.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki D. L., Sawicki S. G. Short-lived minus-strand polymerase for Semliki Forest virus. J Virol. 1980 Apr;34(1):108–118. doi: 10.1128/jvi.34.1.108-118.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar V., Stevens T. M., Schlesinger R. W. Studies on the nature of dengue viruses. II. Characterization of viral RNA and effects of inhibitors of RNA synthesis. Virology. 1966 Oct;30(2):303–312. doi: 10.1016/0042-6822(66)90105-x. [DOI] [PubMed] [Google Scholar]
- Trent D. W., Swensen C. C., Qureshi A. A. Synthesis of Saint Louis encephalitis virus ribonucleic acid in BHK-21-13 cells. J Virol. 1969 Apr;3(4):385–394. doi: 10.1128/jvi.3.4.385-394.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Sedwick W. D., Plotkin S. A., Maes R. Cytopathic effect of rubella virus in RHK21 cells and growth to high titers in suspension culture. Virology. 1965 Oct;27(2):239–241. doi: 10.1016/0042-6822(65)90170-4. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G., Gross H. J. Studies on virus-specific nucleic acids synthesized in vertebrate and mosquito cells infected with flaviviruses. Virology. 1978 Sep;89(2):423–437. doi: 10.1016/0042-6822(78)90185-x. [DOI] [PubMed] [Google Scholar]
- Westaway E. G., McKimm J. L., McLeod L. G. Heterogeneity among flavivirus proteins separated in slab gels. Arch Virol. 1977;53(4):305–312. doi: 10.1007/BF01315629. [DOI] [PubMed] [Google Scholar]