Abstract
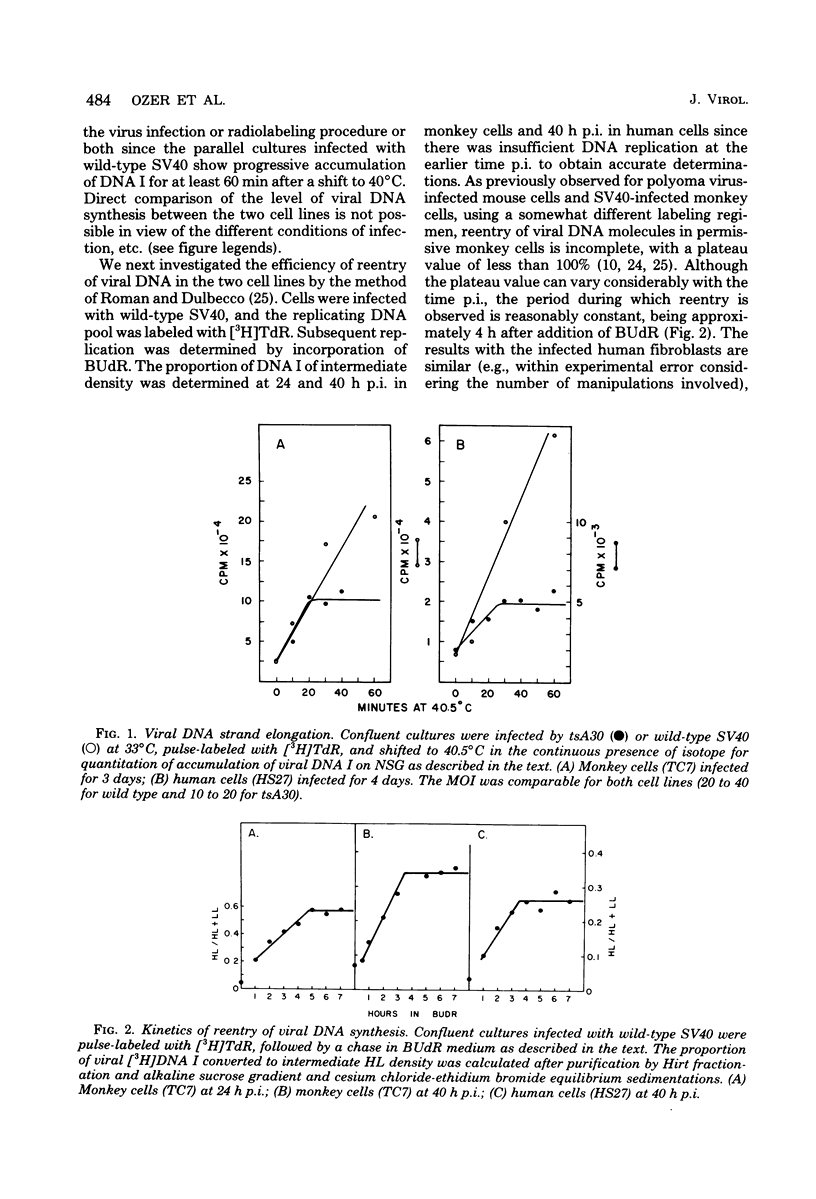
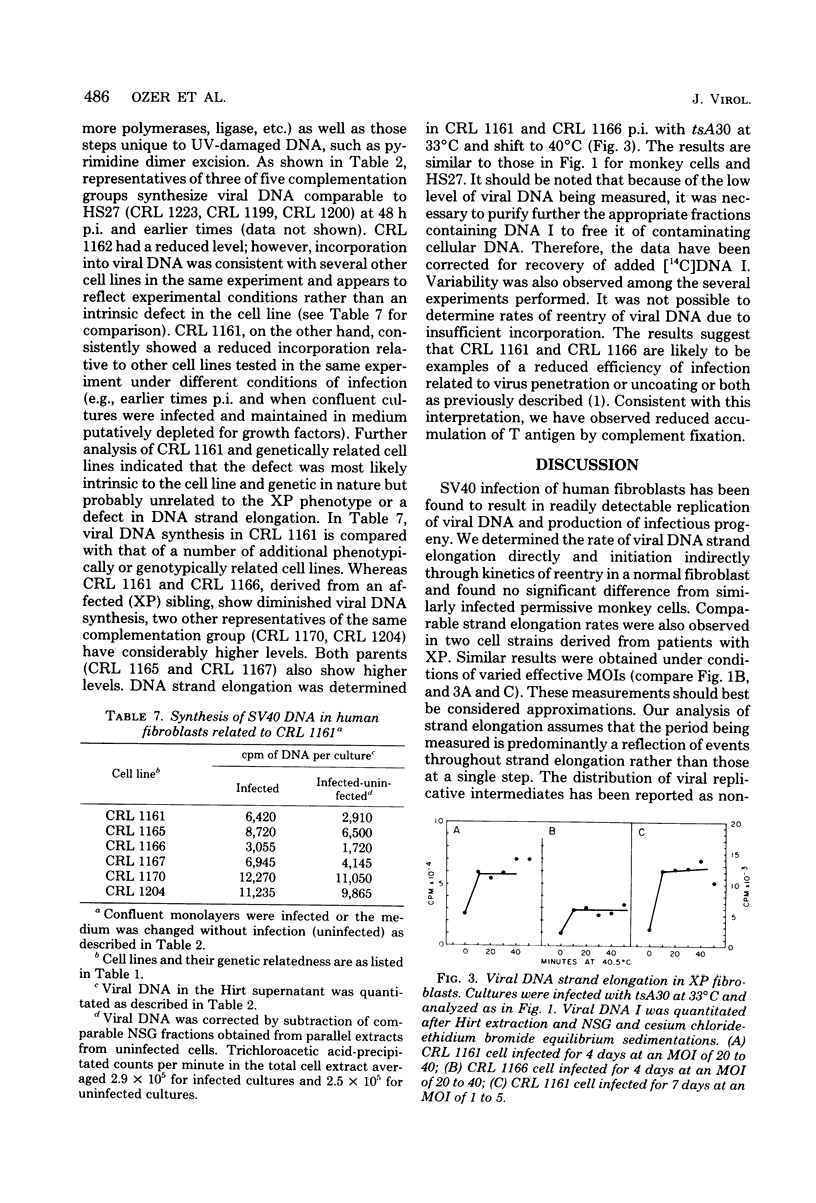
Simian virus 40 infection of semipermissive human diploid fibroblasts (HF), at early passage in cell culture, was compared with that of permissive established monkey cell lines. Viral DNA can be readily detected at 24 to 48 h postinfection at 37 degrees C with a high multiplicity of infection, approaching 10% of that of monkey cells (TC7). The length of time necessary for replication of an average molecule of viral DNA was found to be indistinguishable in HF and TC7 cells. Strand elongation plus termination were assessed by following the accumulation of DNA I at 40 degrees C from replicative intermediates of tsA30 prelabeled at 33 degrees C, obviating isotope pool problems. Combined initiation and elongation of wild-type viral DNA was measured by density shift experiments involving a 5-bromodeoxyuridine chase of prelabeled [3H]thymidine-labeled viral DNA. Determination of accumulation of viral T and V antigens supports the conclusion that the most likely basis for the reduced virus yield in HF cells results from the inefficiency of an early stage in virus infection, before or during uncoating. Similar results were obtained in fibroblasts derived from patients with xeroderma pigmentosum, suggesting that enzymes of UV repair are not required in unirradiated simian virus 40 DNA synthesis.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Susceptibility of human cell strains to transformation by simian virus 40 and simian virus 40 deoxyribonucleic acid. J Virol. 1970 Oct;6(4):470–475. doi: 10.1128/jvi.6.4.470-475.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. SV40 T antigen induction and transformation in human fibroblast cell strains. Virology. 1968 Oct;36(2):254–261. doi: 10.1016/0042-6822(68)90142-6. [DOI] [PubMed] [Google Scholar]
- Blattner W. A., Lubiniecki A. S., Mulvihill J. J., Lalley P., Fraumeni J. F., Jr Genetics of SV40 T-antigen expression: studies of twins, heritable syndromes and cancer families. Int J Cancer. 1978 Sep 15;22(3):231–238. doi: 10.1002/ijc.2910220303. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Gilden R. V. A comparison of the replication cycles of simian virus 40 in human diploid and African green monkey kidney cells. Virology. 1966 Jan;28(1):150–162. doi: 10.1016/0042-6822(66)90316-3. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Berg P. Requirement of a Cytoplasmic Fraction for Synthesis of SV40 Deoxyribonucleic Acid in Isolated Nuclei*. J Biol Chem. 1975 Jun 10;250(11):4348–4354. [PubMed] [Google Scholar]
- Dooley D. C., Ryzlak M. T., Ozer H. L. Endonucleases and simian virus 40 virions: origin of a virion-associated endonuclease. J Virol. 1976 Feb;17(2):352–362. doi: 10.1128/jvi.17.2.352-362.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Hunter T. In vitro polyoma DNA synthesis: requirement for cytoplasmic factors. J Virol. 1975 Jan;15(1):97–107. doi: 10.1128/jvi.15.1.97-107.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARDI A. J., JENSEN F. C., KOPROWSKI H. SV40-INDUCED TRANFORMATION OF HUMAN DIPLOID CELLS: CRISIS AND RECOVERY. J Cell Physiol. 1965 Feb;65:69–83. doi: 10.1002/jcp.1030650110. [DOI] [PubMed] [Google Scholar]
- Green M. H., Brooks T. L. Recently replicated simian virus 40 DNA is a preferential template for transcription and replication. J Virol. 1978 May;26(2):325–334. doi: 10.1128/jvi.26.2.325-334.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand R., German J. A retarded rate of DNA chain growth in Bloom's syndrome. Proc Natl Acad Sci U S A. 1975 Feb;72(2):758–762. doi: 10.1073/pnas.72.2.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kidwell W. R., Saral R., Martin R. G., Ozer H. L. Characterization of an endonuclease associated with simian virus 40 virions. J Virol. 1972 Sep;10(3):410–416. doi: 10.1128/jvi.10.3.410-416.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavialle C., Suarez H. G., Morris A. G., Estrade S., Stévenet J., Cassingena R. Simian virus 40-chinese hamster kidney cell interaction. II. The semipermissivity of the cell system. Arch Virol. 1976;50(1-2):137–146. doi: 10.1007/BF01318008. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Anderson D. E., Smith J. L., Jr, Howell J. B., Krush A. J. Xeroderma pigmentosum, malignant melanoma, and congenital ichthyosis. A family study. Arch Dermatol. 1967 Dec;96(6):625–635. [PubMed] [Google Scholar]
- O'Neill F. J., Carroll D. Appearance of defective simian virus 40 following infection of cultured human glioblastoma cells. Virology. 1978 Jun 1;87(1):109–119. doi: 10.1016/0042-6822(78)90163-0. [DOI] [PubMed] [Google Scholar]
- O'Neill F. J. Propagation of simian virus 40 in a human cell line. Virology. 1976 Jul 1;72(1):287–289. doi: 10.1016/0042-6822(76)90333-0. [DOI] [PubMed] [Google Scholar]
- Ozer H. L. Synthesis and assembly of simian virus 40. I. Differential synthesis of intact virions and empty shells. J Virol. 1972 Jan;9(1):41–51. doi: 10.1128/jvi.9.1.41-51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Takemoto K. K. Site of host restriction of simian virus 40 mutants in an established African green monkey kidney cell line. J Virol. 1969 Oct;4(4):408–415. doi: 10.1128/jvi.4.4.408-415.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., Potter A. M., Oxford J. S. Comparison of transformation and T antigen induction in human cell lines. J Virol. 1970 Mar;5(3):293–298. doi: 10.1128/jvi.5.3.293-298.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakusanova T., Smales W. P., Kaplan J. C., Black P. H. Effect of mitomycin C and 60Co gamma-irradiation on the replication of SV40 in cell lines of varying permissivity for SV40 replication. J Gen Virol. 1979 Apr;43(1):235–239. doi: 10.1099/0022-1317-43-1-235. [DOI] [PubMed] [Google Scholar]
- Robbins J. H., Kraemer K. H., Lutzner M. A., Festoff B. W., Coon H. G. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974 Feb;80(2):221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- Roman A., Dulbecco R. Fate of polyoma form IDNA during replication. J Virol. 1975 Jul;16(1):70–74. doi: 10.1128/jvi.16.1.70-74.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman A. Kinetics of reentry of polyoma progeny form I DNA into replication as a function of time postinfection. Virology. 1979 Jul 30;96(2):660–663. doi: 10.1016/0042-6822(79)90125-9. [DOI] [PubMed] [Google Scholar]
- Seidman M. M., Salzman N. P. Late replicative intermediates are accumulated during simian virus 40 DNA replication in vivo and in vitro. J Virol. 1979 May;30(2):600–609. doi: 10.1128/jvi.30.2.600-609.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. Repair deficient human disorders and cancer. Nature. 1978 Feb 23;271(5647):713–717. doi: 10.1038/271713a0. [DOI] [PubMed] [Google Scholar]
- Slater M. L., Ozer H. L. Temperature-sensitive mutants of Balb/3T3 cells: description of a mutant affected in cellular and polyoma virus DNA synthesis. Cell. 1976 Feb;7(2):289–295. doi: 10.1016/0092-8674(76)90028-3. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Owens R. B., Hiller A. J., Nelson-Rees W. A., Johnston J. O. The biology of human cells in tissue culture. I. Characterization of cells derived from osteogenic sarcomas. Int J Cancer. 1976 Feb 15;17(2):219–234. doi: 10.1002/ijc.2910170211. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


