Abstract
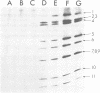
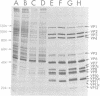
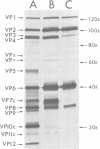
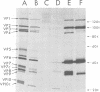
The United Kingdom tissue-adapted bovine rotavirus growing in African green monkey kidney (BSC-1) cells was selected as a model system with which to study the detailed molecular virology of rotavirus replication. Study of the kinetics of infectious virus production revealed a fairly rapid replication cycle, with maximum yield of virus after 10 to 12 h at 37 degrees C. Progeny genome synthesis was first detected during the virus latent period at 2 to 3 h postinfection. Study of the kinetics of viral polypeptide synthesis showed that virus rapidly inhibited cellular polypeptide synthesis such that by 4 h postinfection, only virus-induced polypeptides, 15 of which were detected, were being synthesized. No qualitative changes in the pattern of viral polypeptide synthesis were observed during infection, although, based on kinetic synthesis, three quantitative classes of polypeptides were defined. Pulse-chase analysis revealed three post-translational changes in viral proteins, two of which were shown to be due to glycosylation. Tunicamycin inhibition studies were used to identify the putative non-glycosylated precursors of the two glycoproteins. Comparison of the infected-cell polypeptides with those present in purified virions revealed that mot of the virus-induced proteins were incorporated into virions, with only VP9 being a truly nonstructural protein. Some localization of the various polypeptides within the purified virion was achieved by producing viral cores.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Clark S. M., Barnett B. B., Spendlove R. S. Production of high-titer bovine rotavirus with trypsin. J Clin Microbiol. 1979 Mar;9(3):413–417. doi: 10.1128/jcm.9.3.413-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Ensminger W. D., Tamm I. Cellular DNA and protein synthesis in reovirus-infected L cells. Virology. 1969 Oct;39(2):357–360. doi: 10.1016/0042-6822(69)90062-2. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Gerba C. P., Smith E. M. Simian rotavirus SA11 replication in cell cultures. J Virol. 1979 Sep;31(3):810–815. doi: 10.1128/jvi.31.3.810-815.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewett T. H., Woode G. N. The rotaviruses. Arch Virol. 1978;57(1):1–23. doi: 10.1007/BF01315633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Garon C. F., Wyatt R. G., Mebus C. A., van Kirk D. H., Chanock R. M., Kapikian A. Z. Differentiation of human and calf reoviruslike agents associated with diarrhea using polyacrylamide gel electrophoresis of RNA. Virology. 1976 Oct 1;74(1):86–92. doi: 10.1016/0042-6822(76)90131-8. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Theodore T. S. Polypeptides of simian rotavirus (SA-11) determined by a continuous polyacrylamide gel electrophoresis method. J Gen Virol. 1979 May;43(2):463–466. doi: 10.1099/0022-1317-43-2-463. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Mukoyama A. Polypeptides of bovine rotavirus. J Gen Virol. 1979 May;43(2):309–316. doi: 10.1099/0022-1317-43-2-309. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- McNulty M. S., Allan G. M., McFerran J. B. Cell culture studies with a cytopathic bovine rotavirus. Arch Virol. 1977;54(3):201–209. doi: 10.1007/BF01314786. [DOI] [PubMed] [Google Scholar]
- McNulty M. S. Rotaviruses. J Gen Virol. 1978 Jul;40(1):1–18. doi: 10.1099/0022-1317-40-1-1. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F., Bridger J. C., Woode G. N. Characterisation of a rotavirus.20b. Nature. 1975 Dec 18;258(5536):631–633. doi: 10.1038/258631a0. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J Virol. 1975 Nov;16(5):1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Further biochemical characterization, including the detection of surface glycoproteins, of human, calf, and simian rotaviruses. J Virol. 1977 Oct;24(1):91–98. doi: 10.1128/jvi.24.1.91-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. M., Estes M. K., Graham D. Y., Gerba C. P. A plaque assay for the simian rotavirus SAII. J Gen Virol. 1979 Jun;43(3):513–519. doi: 10.1099/0022-1317-43-3-513. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Thouless M. E. Rotavirus polypeptides. J Gen Virol. 1979 Jul;44(1):187–197. doi: 10.1099/0022-1317-44-1-187. [DOI] [PubMed] [Google Scholar]
- Todd D., McNulty M. S. Biochemical studies on a reovirus-like agent (rotovirus) from lambs. J Virol. 1977 Mar;21(3):1215–1218. doi: 10.1128/jvi.21.3.1215-1218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd D., McNulty M. S. Characterization of pig rotavirus RNA. J Gen Virol. 1976 Oct;33(1):147–150. doi: 10.1099/0022-1317-33-1-147. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J. Multiple forms of SS leads to DS RNA polymerase activity in reovirus-infected cells. Nature. 1974 Feb 1;247(5439):313–315. doi: 10.1038/247313a0. [DOI] [PubMed] [Google Scholar]