Abstract
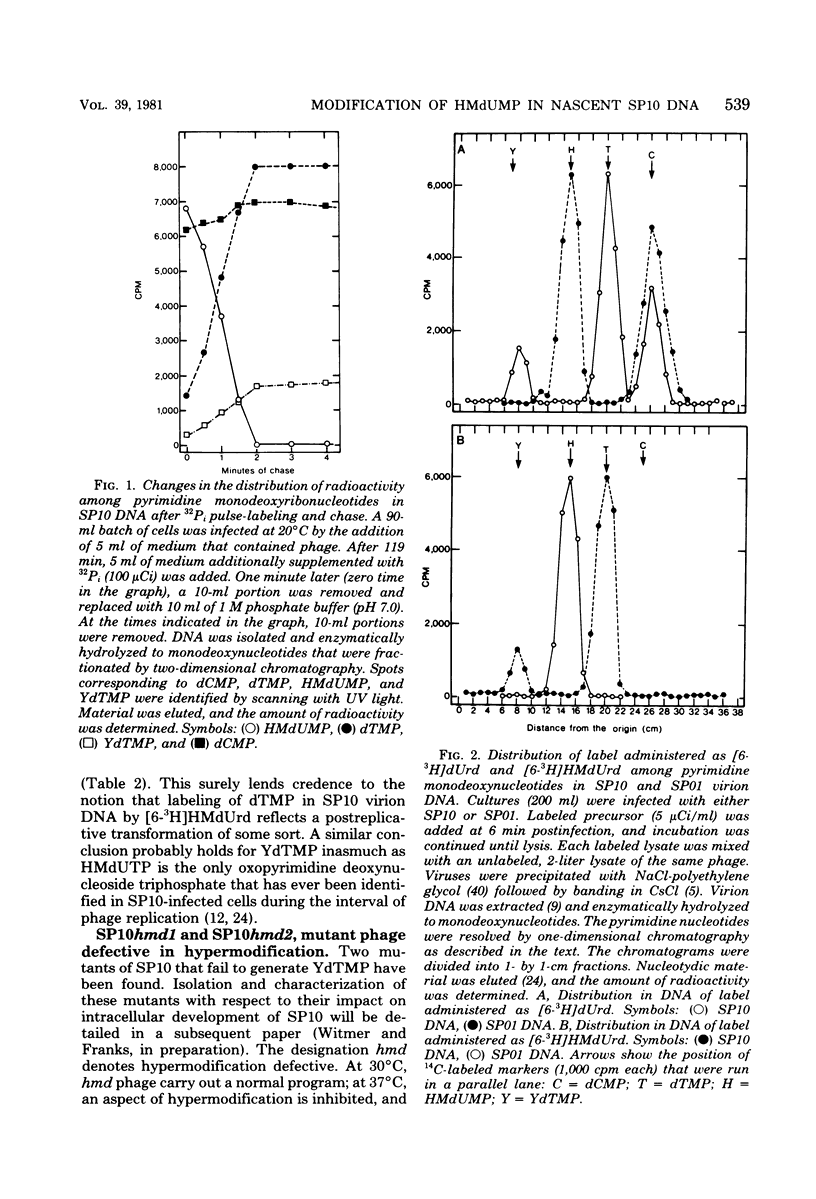
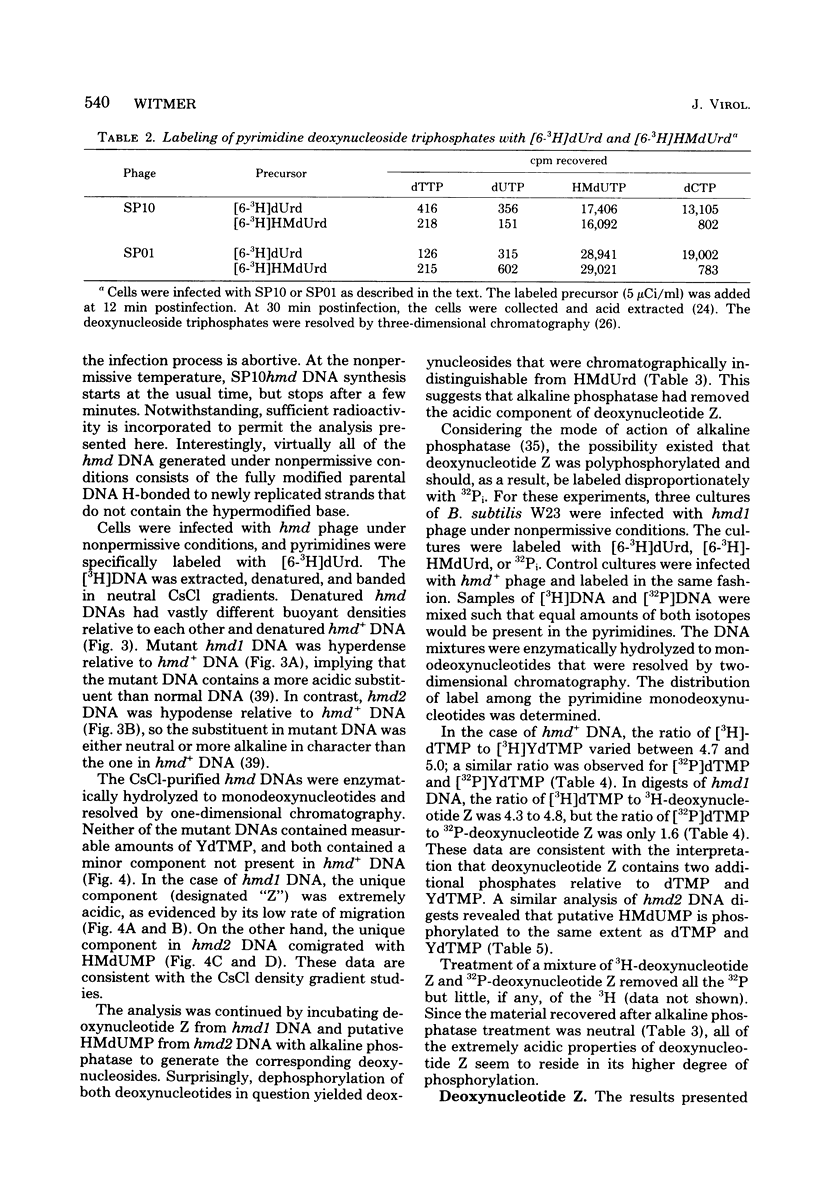
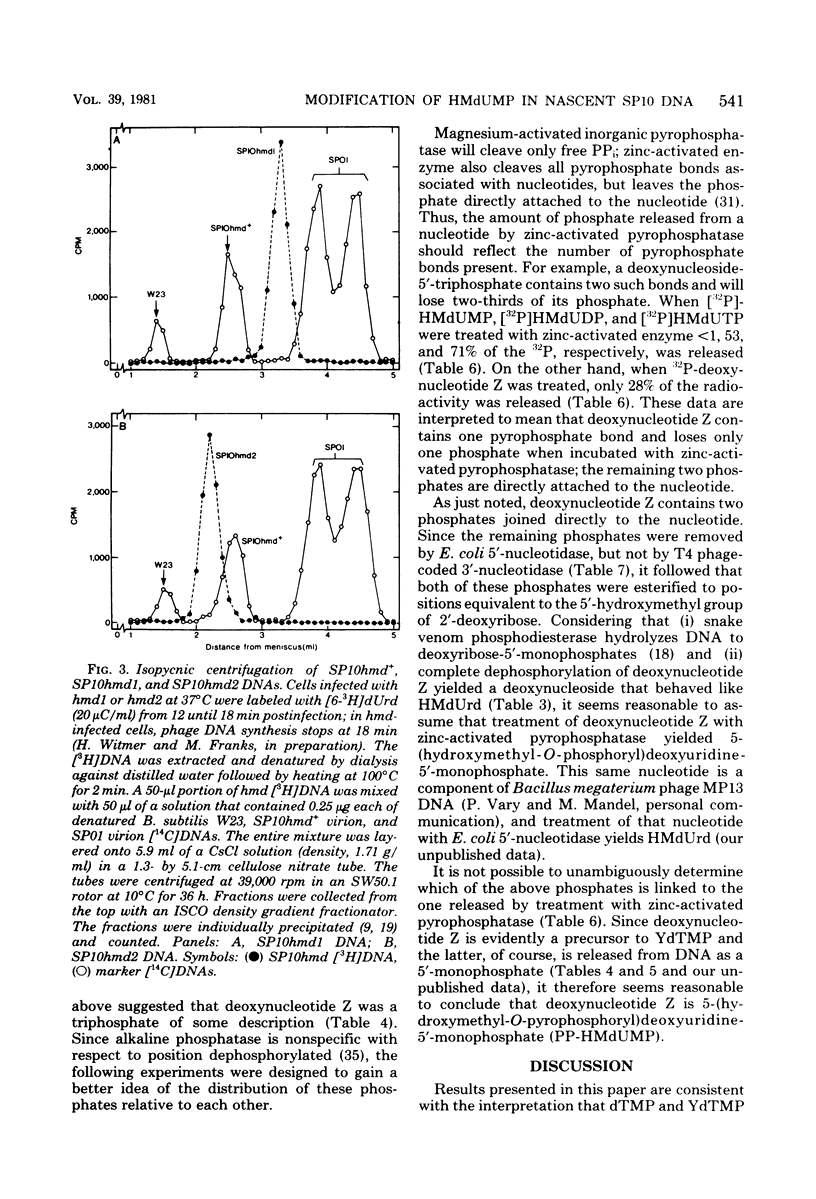
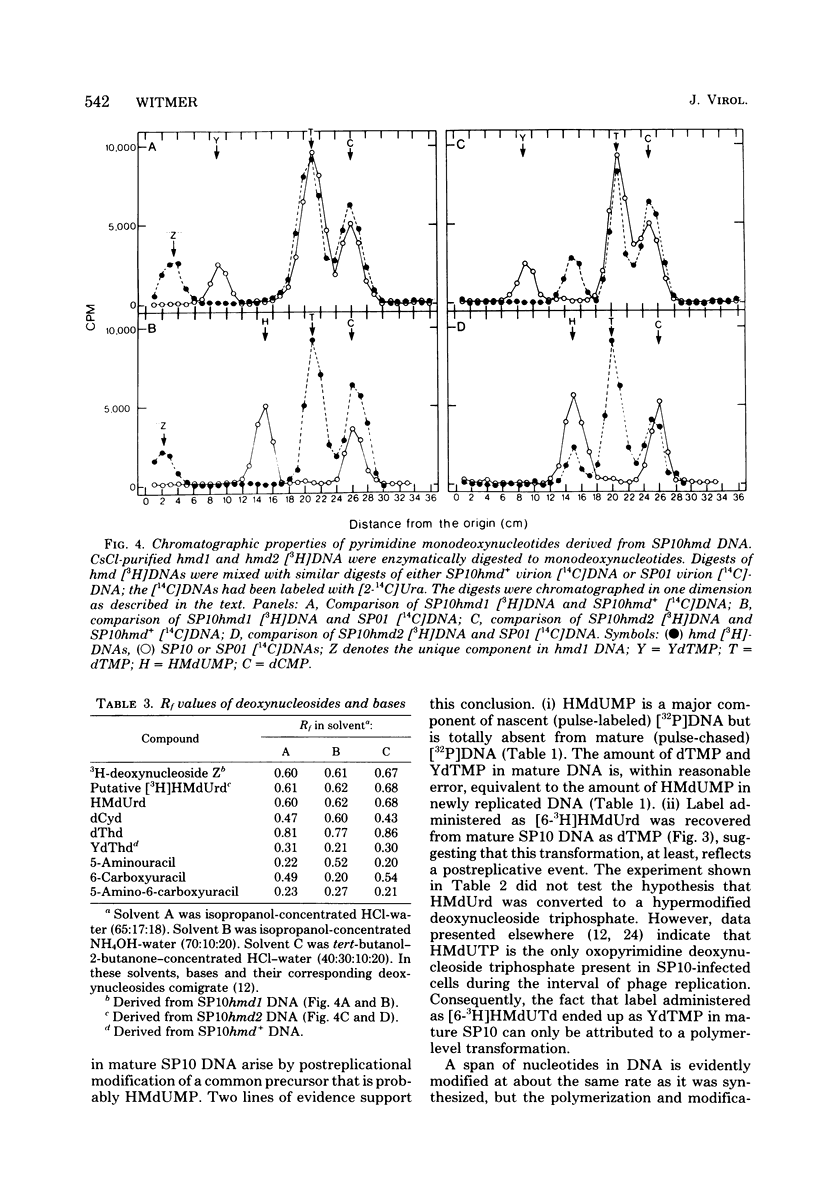
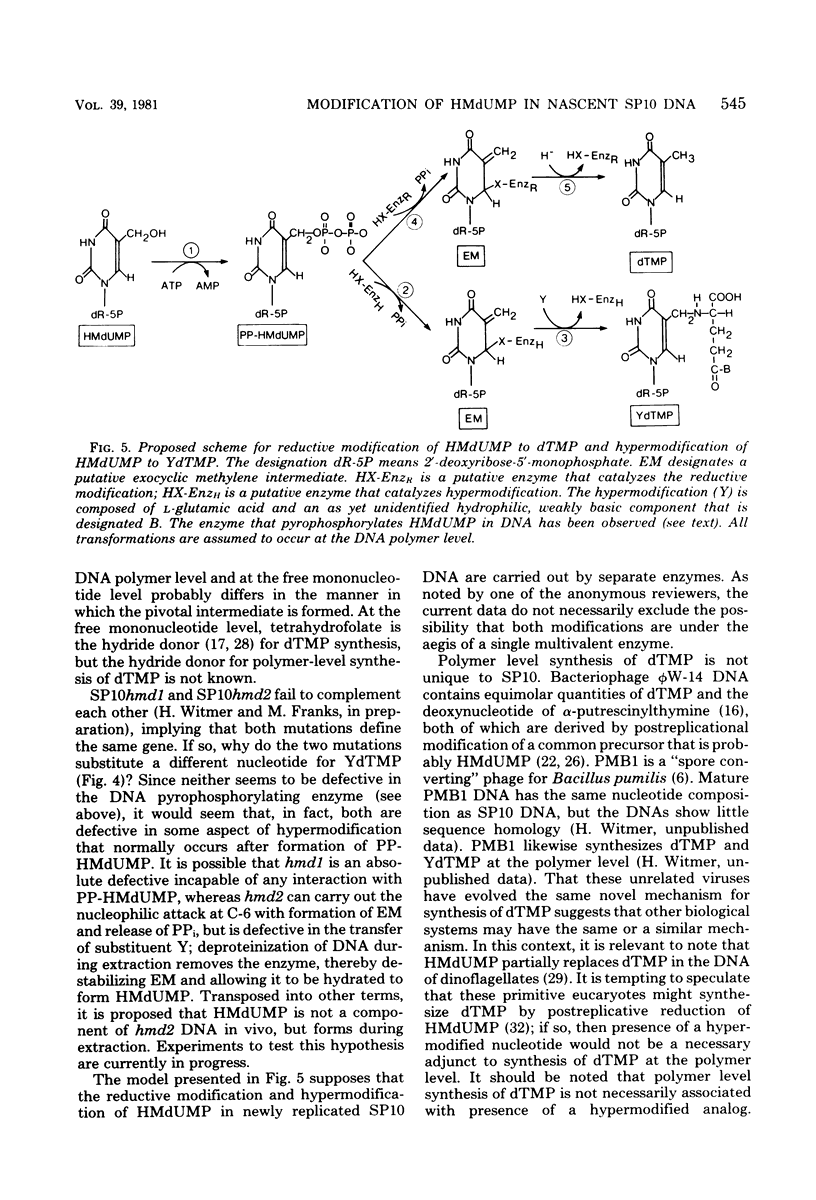
Mature DNA of Bacillus subtilis W23 phage SP10 contains a hypermodified nucleotide (YdTMP) that replaces ca. 20% of the DTMP. SP10 DNA was pulse-labeled for 1 min at 20 degrees C with 32Pi. Among the oxopyrimidine nucleotides, virtually all of the radioactivity was recovered as 5-hydroxymethyldeoxyuridylate (HMdUMP). During the subsequent chase, radioactivity was lost from HMdUMP and recovered as YdTMP. At 37 degrees C, exogenous [6-3H]5-hydroxymethyldeoxyuridine (HMdUrd) was incorporated into SP10 DNA. Label administered as HMdUrd was phosphorylated to HMdUTP in the infected cells, but all radioactivity was recovered from SP10 DNA as YdTMP and dTMP. Two heat-sensitive mutants defective in hypermodification of SP10 DNA are described. In one mutant, HMdUMP replaces YdTMP in DNA. The other mutant generates a DNA containing a novel deoxynucleotide in place of YdTMP. The novel deoxynucleotide seems to consist of PPi esterified to the 5-hydroxymethyl function of HMdUMP (PP-HMdUMP). Both mutants make normal amounts of dTMP. The data are discussed in terms of the following conclusions. (i) Both oxopyrimidine nucleotides in mature SP10 DNA are derived by postreplicative modification of HMdUMP in nascent DNA. (ii) PP-HMdUMP is an intermediate that facilitate formation of a putative exocyclic methylene intermediate which receives the hypermodification. It is also argued that PP-HMdUMP and the same exocyclic methylene intermediate could serve as intermediates in reductive modification to dTMP. (iii) YdTMP is not an intermediate in the formation of dTMP, and reductive modification proceeds independently of hypermodification.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alegria A. H. Hydroxymethylation of pyrimidine mononucleotides with formaldehyde. Biochim Biophys Acta. 1967 Dec 19;149(2):317–324. doi: 10.1016/0005-2787(67)90159-1. [DOI] [PubMed] [Google Scholar]
- Becker A., Hurwitz J. The enzymatic cleavage of phosphate termini from polynucleotides. J Biol Chem. 1967 Mar 10;242(5):936–950. [PubMed] [Google Scholar]
- Bramucci M. G., Keggins K. M., Lovett P. S. Bacteriophage conversion of spore-negative mutants to spore-positive in Bacillus pumilus. J Virol. 1977 Apr;22(1):194–202. doi: 10.1128/jvi.22.1.194-202.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon C., Gallop P. M., Marmur J., Hayashi H., Nakanishi K. Structure of a new pyrimidine from Bacillus subtilis phage SP-15 nucleic acid. Nat New Biol. 1972 Sep 20;239(90):70–71. doi: 10.1038/newbio239070a0. [DOI] [PubMed] [Google Scholar]
- Budman D. R., Pardee A. B. Thymidine and thymine incorporation into deoxyribonucleic acid: inhibition and repression by uridine of thymidine phosphorylase of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1546–1550. doi: 10.1128/jb.94.5.1546-1550.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella E., Markewych O., Dosmar M., Witmer H. Production and expression of dTMP-enriched DNA of bacteriophage SP15. J Virol. 1978 Dec;28(3):753–766. doi: 10.1128/jvi.28.3.753-766.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M., Kalbacher B. The control of ribonucleic acid synthesis in Escherichia coli. V. Characterization of a nucleotide associated with the stringent response. J Biol Chem. 1970 May 10;245(9):2309–2318. [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSSE J., KAISER A. D., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VIII. Frequencies of nearest neighbor base sequences in deoxyribonucleic acid. J Biol Chem. 1961 Mar;236:864–875. [PubMed] [Google Scholar]
- Kropinski A. M., Bose R. J., Warren R. A. 5-(4-Aminobutylaminomethyl)uracil, an unusual pyrimidine from the deoxyribonucleic acid of bacteriophage phiW-14. Biochemistry. 1973 Jan 2;12(1):151–157. doi: 10.1021/bi00725a025. [DOI] [PubMed] [Google Scholar]
- Kunitani M. G., Santi D. V. On the mechanism of 2'-deoxyuridylate hydroxymethylase. Biochemistry. 1980 Apr 1;19(7):1271–1275. doi: 10.1021/bi00548a001. [DOI] [PubMed] [Google Scholar]
- Lembach K. J., Buchanan J. M. The relationship of protein synthesis to early transcriptive events in bacteriophage T4-infected Escherichia coli B. J Biol Chem. 1970 Apr 10;245(7):1575–1587. [PubMed] [Google Scholar]
- Levner M. H., Cozzarelli N. R. Replication of viral DNA in SPO1-infected Bacillus subtilis. I. Replicative intermediates. Virology. 1972 May;48(2):402–416. doi: 10.1016/0042-6822(72)90051-7. [DOI] [PubMed] [Google Scholar]
- Lipmann F., Sy J. The enzymic mechanism of guanosine 5',3'-polyphosphate synthesis. Prog Nucleic Acid Res Mol Biol. 1976;17:1–14. doi: 10.1016/s0079-6603(08)60063-x. [DOI] [PubMed] [Google Scholar]
- Maltman K. L., Neuhard J., Lewis H. A., Warren R. A. Synthesis of thymine and alpha-putrescinylthymine in bacteriophage phi W-14-infected Pseudomonas acidovorans. J Virol. 1980 May;34(2):354–359. doi: 10.1128/jvi.34.2.354-359.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markewych O., Boghosian A., Dosmar M., Ende D., Witmer H. SP-10 bacteriophage-specific nucleic acid and enzyme synthesis in Bacillus subtilis W23. J Virol. 1977 Jan;21(1):84–95. doi: 10.1128/jvi.21.1.84-95.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markewych O., Casella E., Dosmar M., Witmer H. Deoxythymidine nucleotide metabolism in Bacillus subtilis W23 infected with bacteriophage SP1Oc: preliminary evidence that dTMP in SP10c DNA is synthesized by a novel, bacteriophage-specific mechanism. J Virol. 1979 Jan;29(1):61–68. doi: 10.1128/jvi.29.1.61-68.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. I. Purification and properties. J Biol Chem. 1967 Sep 10;242(17):3896–3904. [PubMed] [Google Scholar]
- Neuhard J., Maltman K. L., Warren R. A. Bacteriophage phi W-14-infected Pseudomonas acidovorans synthesizes hydroxymethyldeoxyuridine triphosphate. J Virol. 1980 May;34(2):347–353. doi: 10.1128/jvi.34.2.347-353.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleotide pools of Novikoff rat hepatoma cells growing in suspension culture. I. Kinetics of incorporation of nucleosides into nucleotide pools and pool sizes during growth cycle. J Cell Physiol. 1971 Apr;77(2):213–240. doi: 10.1002/jcp.1040770212. [DOI] [PubMed] [Google Scholar]
- Rae P. M. Hydroxymethyluracil in eukaryote DNA: a natural feature of the pyrrophyta (dinoflagellates). Science. 1976 Dec 3;194(4269):1062–1064. doi: 10.1126/science.988637. [DOI] [PubMed] [Google Scholar]
- SCHLESINGER M. J., COON M. J. Hydrolysis of nucleoside diand triphosphates by crystalline preparations of yeast inorganic pyrophosphatase. Biochim Biophys Acta. 1960 Jun 17;41:30–36. doi: 10.1016/0006-3002(60)90365-6. [DOI] [PubMed] [Google Scholar]
- Saito H., Shibata T., Ando T. Mapping of genes determining nonpermissiveness and host-specific restriction to bacteriophages in Bacillus subtilis Marburg. Mol Gen Genet. 1979 Feb 26;170(2):117–122. doi: 10.1007/BF00337785. [DOI] [PubMed] [Google Scholar]
- Steele R. E., Rae P. M. Ordered distribution of modified bases in the DNA of a dinoflagellate. Nucleic Acids Res. 1980 Oct 24;8(20):4709–4725. doi: 10.1093/nar/8.20.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI I., MARMUR J. Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis. Nature. 1963 Feb 23;197:794–795. doi: 10.1038/197794a0. [DOI] [PubMed] [Google Scholar]
- Walker M. S., Mandel M. Biosynthesis of 5-(4'5'-dihydroxypentyl) uracil as a nucleoside triphosphate in bacteriophage SP15-infected Bacillus subtilis. J Virol. 1978 Feb;25(2):500–509. doi: 10.1128/jvi.25.2.500-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. A. Modified bases in bacteriophage DNAs. Annu Rev Microbiol. 1980;34:137–158. doi: 10.1146/annurev.mi.34.100180.001033. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]


