Abstract
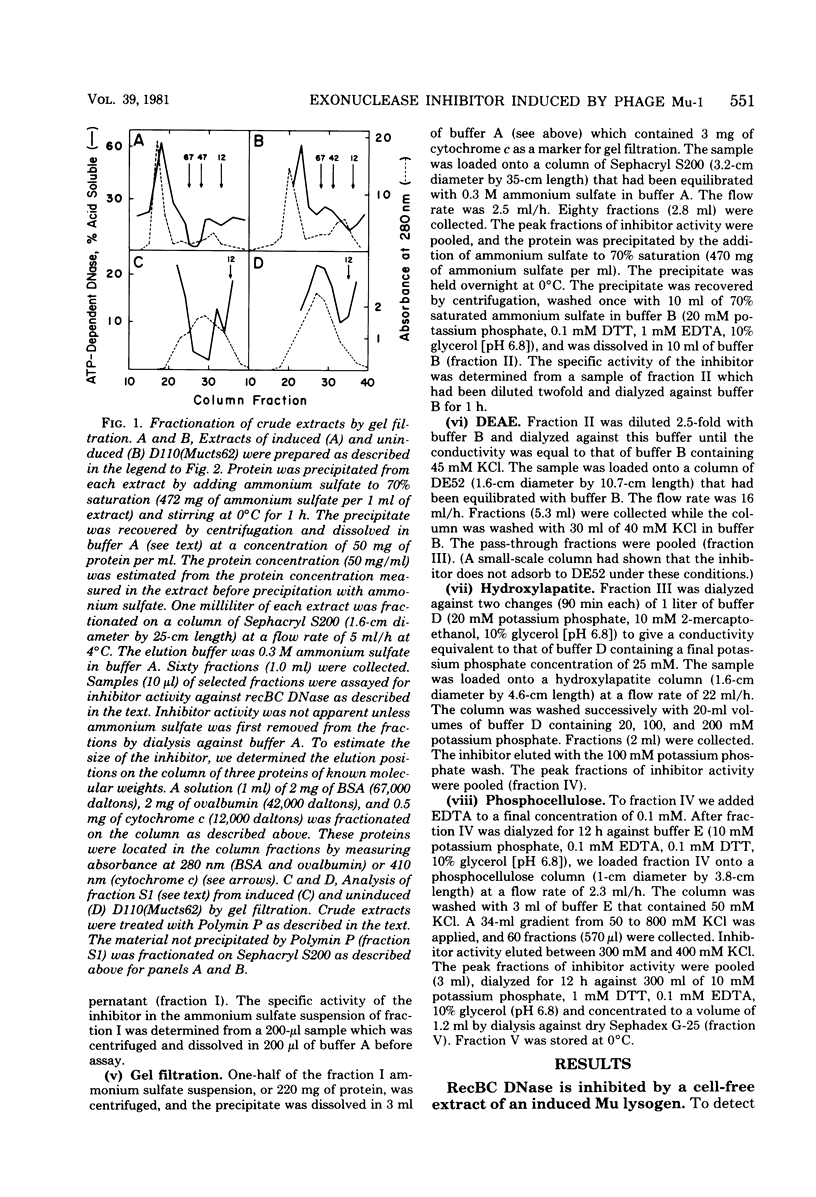
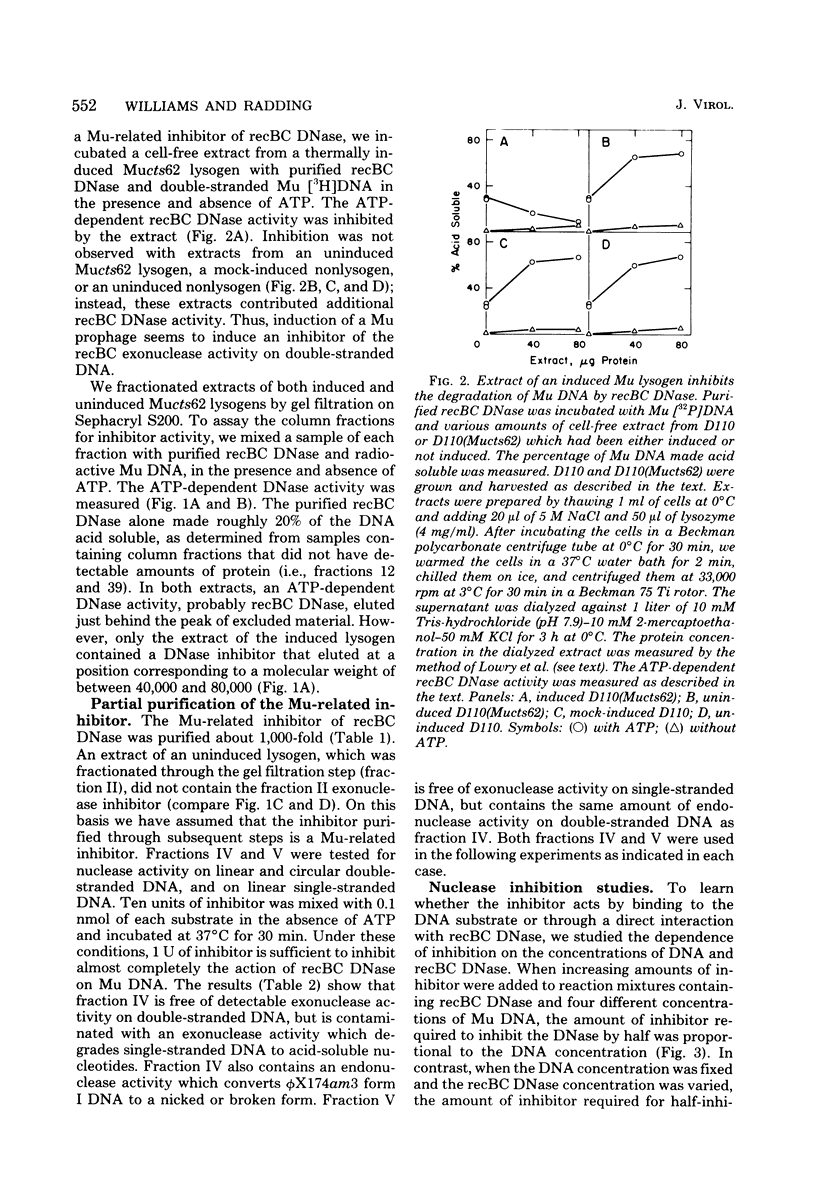
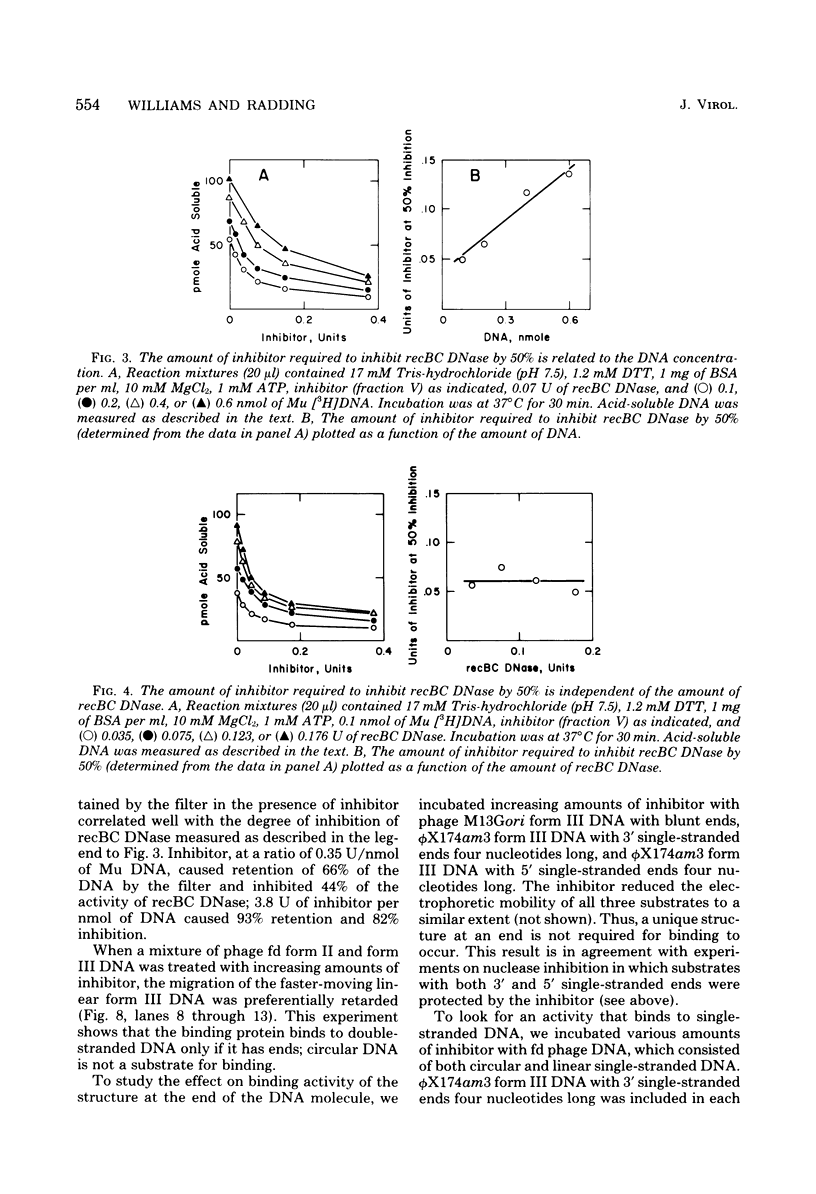
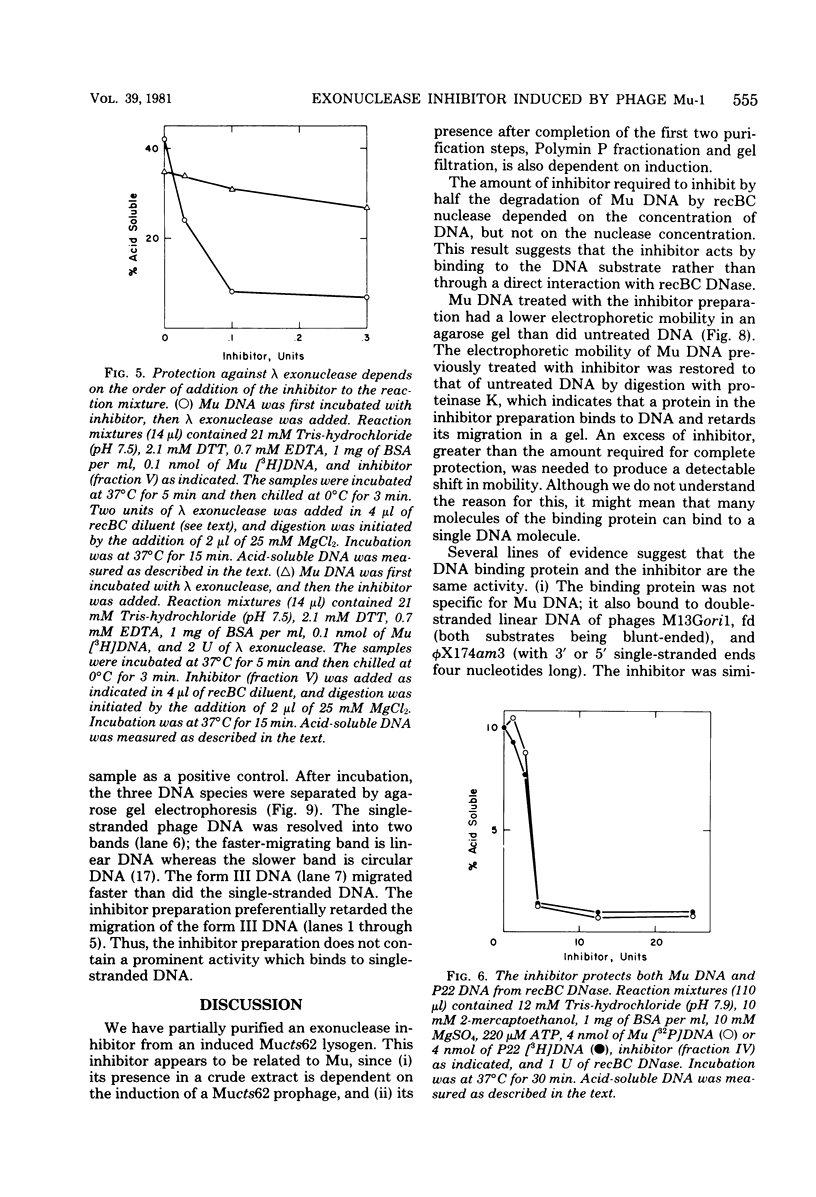
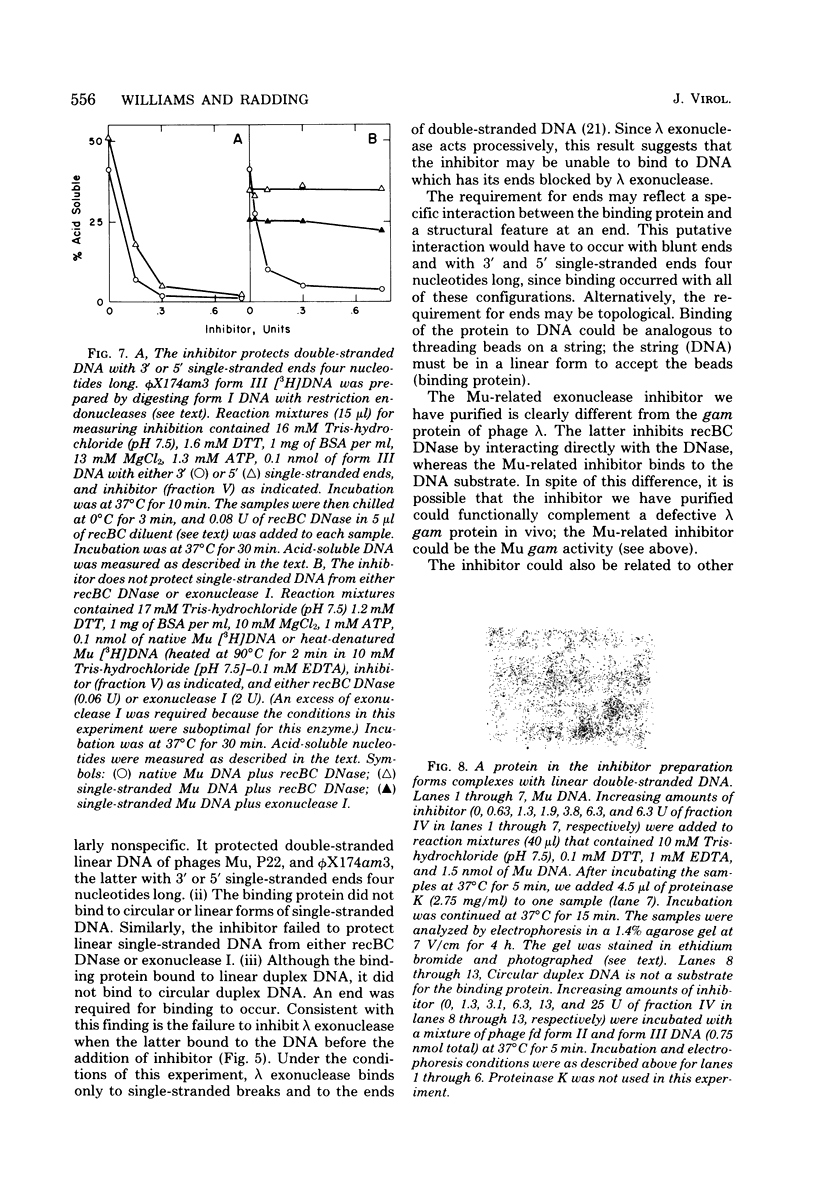
From an induced lysogen of bacteriophage Mu-1, we partially purified a substance of high molecular weight that blocks the action of several exonucleases on double-stranded DNA. The presence of the inhibitor in cell-free extracts is dependent on induction of a Mu prophage. The Mu-related inhibitor acts by binding to double-stranded DNA rather than by interacting with the DNase. The inhibitor protects linear duplex DNA of Mu, P22, and phi X174am3 from exonucleolytic degradation by recBC DNase and lambda exonuclease. Single-stranded DNA, however, is not protected by the inhibitor from degradation by either recBC DNase or exonuclease I. The inhibitor preparation contains a protein that binds to linear duplex DNA, but not to circular duplex DNA; ends are required for binding to occur. Single-stranded DNA is not a substrate for the binding protein. These and other results suggest that the binding protein and the inhibitor are the same activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behme M. T., Lilley G. D., Ebisuzaki K. Postinfection control by bacteriophage T4 of Escherichia coli recBC nuclease activity. J Virol. 1976 Apr;18(1):20–25. doi: 10.1128/jvi.18.1.20-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D. M., Radding C. M. The role of exonuclease and beta protein of phage lambda in genetic recombination. II. Substrate specificity and the mode of action of lambda exonuclease. J Biol Chem. 1971 Apr 25;246(8):2502–2512. [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Cunningham R. P., DasGupta C., Shibata T., Radding C. M. Homologous pairing in genetic recombination: recA protein makes joint molecules of gapped circular DNA and closed circular DNA. Cell. 1980 May;20(1):223–235. doi: 10.1016/0092-8674(80)90250-0. [DOI] [PubMed] [Google Scholar]
- DasGupta C., Shibata T., Cunningham R. P., Radding C. M. The topology of homologous pairing promoted by RecA protein. Cell. 1980 Nov;22(2 Pt 2):437–446. doi: 10.1016/0092-8674(80)90354-2. [DOI] [PubMed] [Google Scholar]
- Feiss M., Margulies T. On maturation of the bacteriophage lambda chromosome. Mol Gen Genet. 1973 Dec 31;127(4):285–295. doi: 10.1007/BF00267099. [DOI] [PubMed] [Google Scholar]
- Giphart-Gassler M., Van de Putte P. Thermo-inducible expression of cloned early genes of bacteriophage Mu. Gene. 1979 Sep;7(1):33–50. doi: 10.1016/0378-1119(79)90041-6. [DOI] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. Purification and properties of the recBC DNase of Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1849–1860. [PubMed] [Google Scholar]
- Kaguni J., Ray D. S. Cloning of a functional replication origin of phage G4 into the genome of phage M13. J Mol Biol. 1979 Dec 25;135(4):863–878. doi: 10.1016/0022-2836(79)90516-3. [DOI] [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martuscelli J., Taylor A. L., Cummings D. J., Chapman V. A., DeLong S. S., Cañedo L. Electron microscopic evidence for linear insertion of bacteriophage MU-1 in lysogenic bacteria. J Virol. 1971 Oct;8(4):551–563. doi: 10.1128/jvi.8.4.551-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacumbaba R., Center M. S. Partial purification and properties of a bacteriophage T7 inhibitor of the host exonuclease V activity. J Virol. 1975 Nov;16(5):1200–1207. doi: 10.1128/jvi.16.5.1200-1207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M., Carter D. M. The role of exonuclease and beta protein of phage lambda in genetic recombination. 3. Binding to deoxyribonucleic acid. J Biol Chem. 1971 Apr 25;246(8):2513–2518. [PubMed] [Google Scholar]
- Sakaki Y. Inactivation of the ATP-dependent DNase of Escherichia coli after infection with double-stranded DNA phages. J Virol. 1974 Dec;14(6):1611–1612. doi: 10.1128/jvi.14.6.1611-1612.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Gudas L. J., Pardee A. B. Degradation of Escherichia coli DNA: evidence for limitation in vivo by protein X, the recA gene product. Mol Gen Genet. 1979 Jan 5;168(1):69–80. doi: 10.1007/BF00267935. [DOI] [PubMed] [Google Scholar]
- Schaus N. A., Wright A. Inhibition of Escherichia coli exonuclease V by bacteriophage Mu. Virology. 1980 Apr 15;102(1):214–217. doi: 10.1016/0042-6822(80)90083-5. [DOI] [PubMed] [Google Scholar]
- Shibata T., Cunningham R. P., DasGupta C., Radding C. M. Homologous pairing in genetic recombination: complexes of recA protein and DNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5100–5104. doi: 10.1073/pnas.76.10.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein J. L., Goldberg E. B. T4 DNA injection. II. Protection of entering DNA from host exonuclease V. Virology. 1976 Jul 1;72(1):212–223. doi: 10.1016/0042-6822(76)90324-x. [DOI] [PubMed] [Google Scholar]
- Skalka A. M. DNA replication--bacteriophage lambda. Curr Top Microbiol Immunol. 1977;78:201–237. [PubMed] [Google Scholar]
- Van Vliet F., Couturier M., De Lafonteyne J., Jedlicki E. Mu-1 directed inhibition of DNA breakdown in Escherichia coli, recA cells. Mol Gen Genet. 1978 Aug 4;164(1):109–112. doi: 10.1007/BF00267606. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Alton N. K., Bassett C. L., Kushner S. R. Amplification in Escherichia coli of enzymes involved in genetic recombination: construction of hybrid ColE1 plasmids carrying the structural gene for exonuclease I. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3492–3496. doi: 10.1073/pnas.73.10.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil E., Rosner A. Effect of adenosine and deoxyadenosine on the incorporation and breakdown of thymidine in Escherichia coli K-12. J Bacteriol. 1970 Aug;103(2):417–421. doi: 10.1128/jb.103.2.417-421.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Putte P., Westmaas G. C., Wijffelman C. Transfection with Mu-DNA. Virology. 1977 Aug;81(1):152–159. doi: 10.1016/0042-6822(77)90067-8. [DOI] [PubMed] [Google Scholar]