Abstract
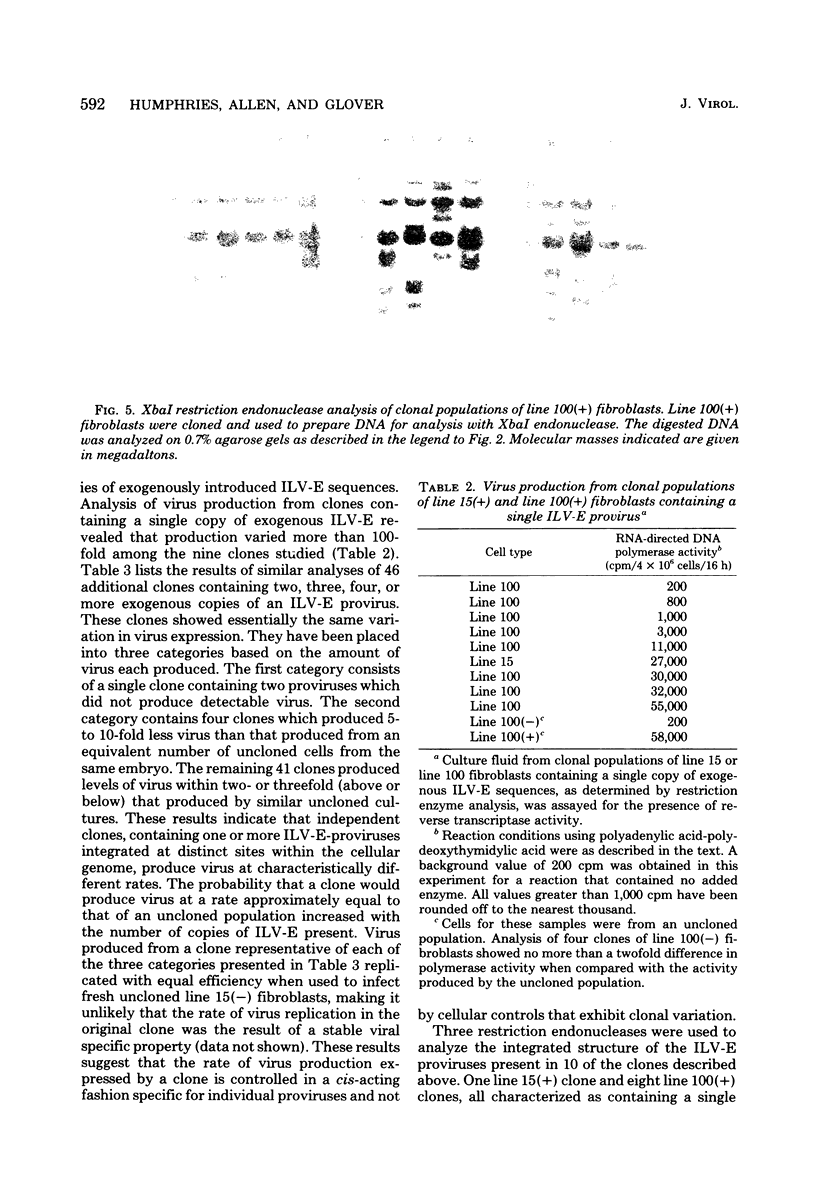
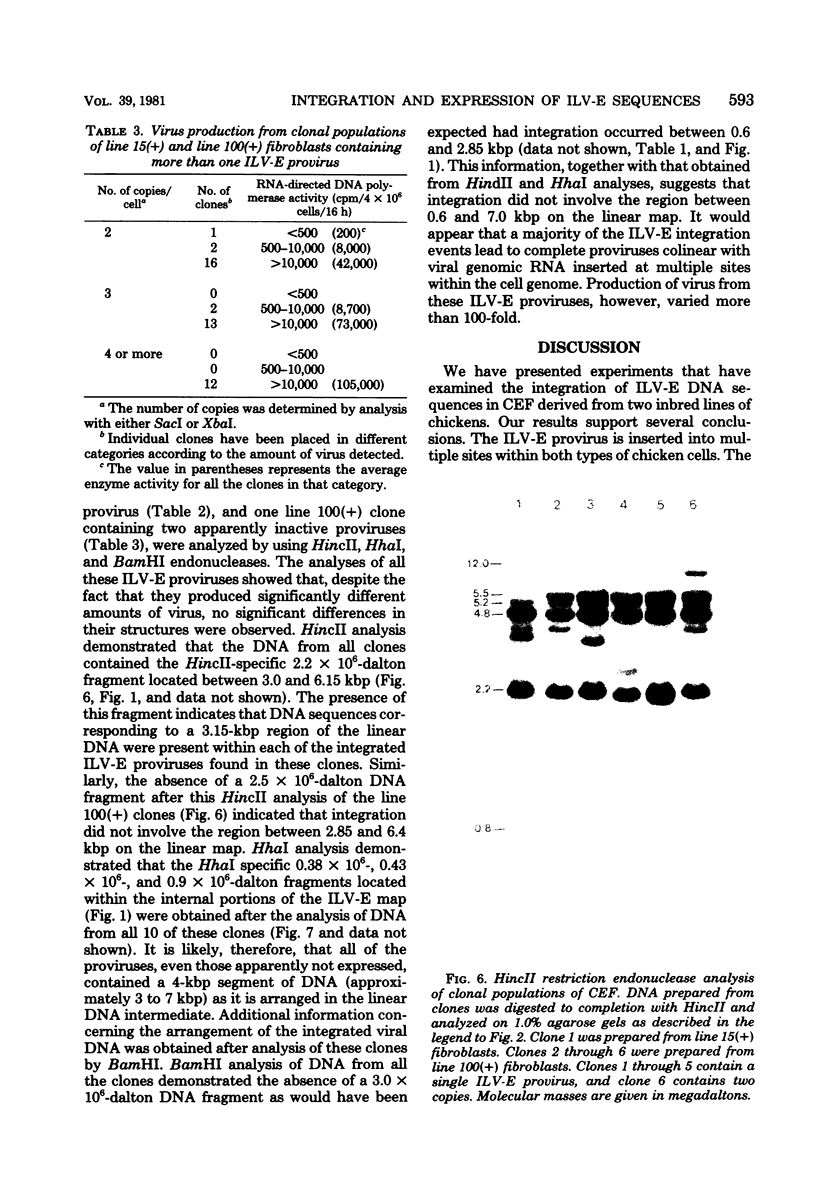
Rous-associated virus-0 is one of several endogenous avian retroviruses that are transmitted vertically and that can be isolated from different inbred lines of chickens. These viruses, referred to here as induced-leukosis viruses bearing a subgroup E glycoprotein (ILV-E), are all closely related. Clonal populations of fibroblasts from line 15B and line 100 inbred chickens have been examined for the presence and expression of exogenously acquired ILV-E sequences. Restriction enzyme analysis of uniform populations of line 15B fibroblasts, prepared by cloning cells either before or after infection with ILV-E, indicates that viral sequences were inserted at multiple sites within the cell genome. Analysis of 49 clonal populations of line 100 fibroblasts containing between one and five copies of exogenous ILV-E sequences demonstrated that each clone was characterized by a unique set of viral DNA insertions within the cell genome. The expression of the exogenous ILV-E sequences within these fibroblast clones was examined by using reverse transcriptase activity as a measure of virus production. Some clones produced an amount of virus equivalent to that produced by an equal number of the uncloned ILV-E-infected parental fibroblasts. Other clones produced 5- to 10-fold less virus. Still other clones produced no detectable virus at all. Among nine clones derived from cells containing a single copy of the ILV-E provirus, the level of virus production differed more than 100-fold. DNA from these clones was analyzed with several different restriction endonucleases to characterize the location and arrangement of the ILV-E sequences. All nine clones consisted of cells that appeared to contain a complete provirus inserted (i) in a different site within the cellular DNA and (ii) in an orientation that was colinear with the viral genomic RNA. It was observed that several cleavage sites potentially affected by methylation were equally available for cleavage in all clones regardless of the level of viral production.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M., Crittenden L. B., Buss E. G. Ev 2, a genetic locus containing structural genes for endogenous virus, codes for Rous-associated virus type 0 produced by line 72 chickens. J Virol. 1980 Jan;33(1):250–255. doi: 10.1128/jvi.33.1.250-255.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrin S. M. Endogenous viral genes of the White Leghorn chicken: common site of residence and sites associated with specific phenotypes of viral gene expression. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5941–5945. doi: 10.1073/pnas.75.12.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Graf T. Isolation of clonal strains of chicken embryo fibroblasts. Exp Cell Res. 1977 Jul;107(2):417–428. doi: 10.1016/0014-4827(77)90363-9. [DOI] [PubMed] [Google Scholar]
- Bird A. P., Taggart M. H., Smith B. A. Methylated and unmethylated DNA compartments in the sea urchin genome. Cell. 1979 Aug;17(4):889–901. doi: 10.1016/0092-8674(79)90329-5. [DOI] [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Lack of infectivity of the endogenous avian leukosis virus-related genes in the DNA of uninfected chicken cells. J Virol. 1976 Feb;17(2):422–430. doi: 10.1128/jvi.17.2.422-430.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden L. B., Motta J. V., Smith E. J. Genetic control of RAV-O production in chickens. Virology. 1977 Jan;76(1):90–97. doi: 10.1016/0042-6822(77)90285-9. [DOI] [PubMed] [Google Scholar]
- Crittenden L. B., Smith E. J., Weiss R. A., Sarma P. S. Host gene control of endogenous avian leukosis virus production. Virology. 1974 Jan;57(1):128–138. doi: 10.1016/0042-6822(74)90114-7. [DOI] [PubMed] [Google Scholar]
- Dhar R., McClements W. L., Enquist L. W., Vande Woude G. F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E., Temin H. M. Formation and structure of infectious DNA of spleen necrosis virus. J Virol. 1977 Jan;21(1):119–130. doi: 10.1128/jvi.21.1.119-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer T. M., Parsons J. T. Analysis of cellular integration sites in avian sarcoma virus infected duck embryo cells. J Virol. 1979 Dec;32(3):762–769. doi: 10.1128/jvi.32.3.762-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntaka R. V., Rao P. Y., Mitsialis S. A., Katz R. Modification of avian sarcoma proviral DNA sequences in nonpermissive XC cells but not in permissive chicken cells. J Virol. 1980 May;34(2):569–572. doi: 10.1128/jvi.34.2.569-572.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishinuma F., DeBona P. J., Astrin S., Skalka A. M. Nucleotide sequence of acceptor site and termini of integrated avian endogenous provirus ev1: integration creates a 6 bp repeat of host DNA. Cell. 1981 Jan;23(1):155–164. doi: 10.1016/0092-8674(81)90280-4. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Vogt P. K., Stubblefield E., Bishop J. M., Varmus H. E. Integration of avian sarcoma virus DNA in chicken cells. Virology. 1981 Jan 15;108(1):208–221. doi: 10.1016/0042-6822(81)90539-0. [DOI] [PubMed] [Google Scholar]
- Humphries E. H., Glover C., Weiss R. A., Arrand J. R. Differences between the endogenous and exogenous DNA sequences of Rous-associated virus-O. Cell. 1979 Nov;18(3):803–815. doi: 10.1016/0092-8674(79)90133-8. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Cooper G. M. Integration, expression, and infectivity of exogenously acquired proviruses of Rous-associated virus-O. J Virol. 1980 Dec;36(3):684–691. doi: 10.1128/jvi.36.3.684-691.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G., Skalka A. M. Nucleotide sequence analysis of the long terminal repeat (LTR) of avian retroviruses: structural similarities with transposable elements. Cell. 1980 Nov;22(2 Pt 2):379–386. doi: 10.1016/0092-8674(80)90348-7. [DOI] [PubMed] [Google Scholar]
- Keshet E., O'Rear J. J., Temin H. M. DNA of noninfectious and infectious integrated spleen necrosis virus (SNV) is colinear with unintegrated SNV DNA and not grossly abnormal. Cell. 1979 Jan;16(1):51–61. doi: 10.1016/0092-8674(79)90187-9. [DOI] [PubMed] [Google Scholar]
- Linial M., Neiman P. E. Infection of chick cells by subgroup E viruses. Virology. 1976 Sep;73(2):508–520. doi: 10.1016/0042-6822(76)90412-8. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- O'Rear J. J., Mizutani S., Hoffman G., Fiandt M., Temin H. M. Infectious and noninfectious recombinant clones of the provirus of SNV differ in cellular DNA and are apparently the same in viral DNA. Cell. 1980 Jun;20(2):423–430. doi: 10.1016/0092-8674(80)90628-5. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Astrin S. M., Salazar F. H. V-15B, an allele of chickens for the production of a noninfectious avian leukosis virus. Virology. 1979 Nov;99(1):10–20. doi: 10.1016/0042-6822(79)90032-1. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Eisenman R., Senior A., Ripley S. Low freqeuncy production of recombinant subgroup E avian leukosis viruses by uninfected V-15B chicken cells. Virology. 1979 Nov;99(1):21–30. doi: 10.1016/0042-6822(79)90033-3. [DOI] [PubMed] [Google Scholar]
- Robinson H. L. Inheritance and expression of chicken genes that are related to avian leukosis sarcoma virus genes. Curr Top Microbiol Immunol. 1978;83:1–36. doi: 10.1007/978-3-642-67087-9_1. [DOI] [PubMed] [Google Scholar]
- Robinson H. L. Intracellular restriction on the growth of induced subgroup E avian type C viruses in chicken cells. J Virol. 1976 Jun;18(3):856–866. doi: 10.1128/jvi.18.3.856-866.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Swanson C. A., Hruska J. F., Crittenden L. B. Production of unique C-type viruses by chicken cells grown in bromodeoxyuridine. Virology. 1976 Jan;69(1):63–74. doi: 10.1016/0042-6822(76)90194-x. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Varmus H. E. Restriction endonuclease mapping of the DNA of Rous-associated virus O reveals extensive homology in structure and sequence with avian sarcoma virus DNA. Virology. 1981 Jan 15;108(1):177–188. doi: 10.1016/0042-6822(81)90537-7. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Mizutani S., Temin H. M. Sequence of retrovirus provirus resembles that of bacterial transposable elements. Nature. 1980 Jun 19;285(5766):550–554. doi: 10.1038/285550a0. [DOI] [PubMed] [Google Scholar]
- Smith E. J., Crittenden L. B., Brinsfield T. H., Jr Status of the endogenous avian leukosis virus in resistant cells from a producing line. Virology. 1974 Oct;61(2):594–596. doi: 10.1016/0042-6822(74)90293-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., de Crombrugghe B., Pastan I. Identification of a functional promoter in the long terminal repeat of Rous sarcoma virus. Cell. 1980 Dec;22(3):787–797. doi: 10.1016/0092-8674(80)90555-3. [DOI] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]