Abstract
In an unusual paradox, asthmatics who are chronically treated with bronchodilating β-agonists sometimes experience a worsening of their condition. A new study describes one possible mechanism and reveals a potential new therapeutic target in the treatment of asthma.
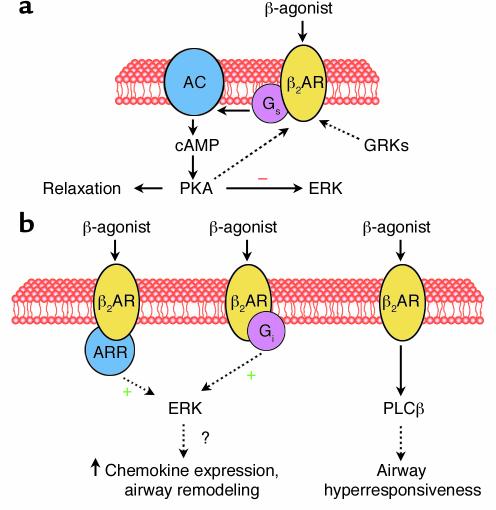
Inhaled selective β2-agonists are the most widely used treatment for the acute relief of asthma symptoms. In patients with asthma, these agents cause bronchodilation (improvement in lung mechanics) and bronchoprotection (reduced responsiveness to nonspecific contractile stimuli). These actions result from binding to the β2-adrenergic receptor (β2AR), a heptahelical receptor that couples predominantly to the stimulatory G protein, Gs (Figure 1a). Once activated by receptor-ligand binding, the α subunit of Gs activates adenylyl cyclase (AC), resulting in cAMP formation. cAMP phosphorylates protein kinase A (PKA), which phosphorylates multiple proteins, leading to reductions in intracellular calcium, smooth muscle relaxation, and bronchodilation.
Figure 1.
(a) Mechanism of β-agonist–induced airway smooth muscle relaxation. Ligand binding to the β2AR activates Gs, leading to adenylyl cyclase (AC) activation, cAMP formation, and subsequent protein kinase A (PKA) activation. PKA phosphorylation of target proteins leads to smooth muscle relaxation and may also inhibit ERK activation. PKA also phosphorylates the β2AR, leading to increased Gi coupling. In addition, ligand binding causes G protein receptor kinase (GRK) phosphorylation of the β2AR, recruiting β-arrestin (ARR). (b) Inflammatory events in the asthmatic airway or regular β-agonist use may augment Gi coupling and/or increase β-arrestin binding. Under these circumstances, β-agonists may result in ERK activation, potentially amplifying production of inflammatory cytokines and leading to airway remodeling. Persistent activation of the β2AR may also lead to phospholipase C-β (PLCβ) expression and consequent airway hyperresponsiveness (8).
It is possible to have too much of a good thing. Despite the ability of β-agonists to effect immediate reversal of airway obstruction, there has been continuing concern that regular use of these drugs may be associated with adverse outcomes. In some, but not all, studies, regularly scheduled use (e.g., multiple times per day, every day) of inhaled β-agonists has resulted in loss of asthma control, declines in morning peak flow, longer durations of asthma exacerbations, and rebound airway hyperresponsiveness (1–6). These adverse effects appear to be particularly important with β-agonists of high intrinsic efficacy, like fenoterol, and in patients with certain β2AR genotypes.
The common explanation for these observations is that the adverse effects of regular β-agonist therapy are related to desensitization of the β2AR (2, 3, 5, 7). The data reported by McGraw et al. in this issue of the JCI (8) suggest an alternative explanation, namely that persistent high-level activation of the β2AR leads to increased expression of phospholipase C-β (PLCβ) in airway smooth muscle. Since agonists such as acetylcholine, histamine, and leukotrienes that cause airway smooth muscle contraction do so by acting on receptors that couple to Gq and activate PLCβ, chronic β-agonists might augment the effects of these bronchoconstrictors. How could this occur? PLCβ hydrolyzes phosphatidylinositol 4,5 biphosphate, resulting in inositol 1,4,5 trisphosphate (IP3) production. IP3 increases intracellular calcium, leading to activation of myosin light chain kinase, myosin phosphorylation, and consequent muscle contraction. If the observations of McGraw et al. are borne out, this mechanism could explain the adverse effects of chronic stimulation of the β2AR. However, before we embrace this hypothesis, which is based on data from mouse airways, it will be important to confirm the observations of McGraw et al. in human airway smooth muscle stimulated with β-agonists rather than by β2AR overexpression.
We think that there may be additional explanations for the bronchoconstrictor effects of chronic β2AR stimulation. For example, in some cell types the β2AR can couple to Gi as well as Gs (9–12). The switch from Gs to Gi coupling appears to be regulated by PKA phosphorylation of the β2AR (13). β2AR-induced Gi activation leads to activation of the extracellular signal–regulated kinase (ERK) MAPKs (Figure 1b) through a pathway dependent on the Gi βγ subunits and activation of Ras (12). β2AR activation also leads to G protein receptor kinase–induced (GRK-induced) phosphorylation of the β2AR, resulting in its interaction with β-arrestin. β-arrestin can serve as a scaffolding protein linking the β2AR to both the ERK and JNK MAPK pathways (9, 14). To complicate the matter, Gs-dependent formation of cAMP by β2AR activation can also inhibit ERK activation (10), apparently as a result of Raf-1 phosphorylation by PKA (15). The ultimate effect of β2AR activation on ERK phosphorylation is likely to reflect a balance of these various events.
Although these pathways have been described in other cell types, none of these events has been examined in airway smooth muscle cells. Thus it is interesting to consider the potential functional implications of β-agonist–induced ERK activation in these cells. Persistent ERK activation is known to be required for mitogenesis in airway smooth muscle cells (16). Activation of ERK is also required for the full expression by airway smooth muscle of the eosinophil chemotactic factors eotaxin and RANTES, as well as other chemokines (17). β-agonists normally inhibit mitogenesis (18) and inhibit expression of eotaxin (19) in airway smooth muscle. However, there may be conditions in which this is not the case. For example, Gs-to-Gi switching could be exaggerated by inflammatory cytokines, which have been shown to increase Gi expression (20). Inflammatory cytokines have also been shown to increase GRK expression in these cells (20). Increased GRK activation could be expected to enhance ERK activation through effects on β-arrestin binding to the β2AR. It is also possible that the balance of ERK-activating and ERK-inhibiting effects of β2AR activation may be affected by β2AR desensitization under conditions of regular β-agonist use.
It is interesting to note that the adverse effects of regular β-agonist use can be observed even weeks after their withdrawal, at a time when β2AR desensitization should be resolved (2). If regular use of β-agonists negatively impacts the balance of factors contributing to airway smooth muscle mitogenesis or airway remodeling in asthma, it could have long-term consequences. Importantly, studies of regular use of β-agonist have reported adverse effects only in subjects homozygous for the Arg16 variant of the β2AR (2, 3, 5). The impact of β2AR polymorphisms on non–Gs-mediated events such as ERK phosphorylation has not been examined in any cell type.
β-agonists are currently one of the most important forms of therapy for asthma. Little is known about non–Gs-mediated effects of β2AR activation and other events that could negatively impact the function of airway smooth muscle in asthma. In this issue of the JCI, McGraw et al. (8) report one such event, induction of PLCβ, but it is possible that this is just the tip of the iceberg. Understanding the panoply of β2AR-mediated events in airway smooth muscle might lead to the design of new agonists that avoid negative effects of β2AR activation while enhancing events that lead to relaxation.
Footnotes
See the related article beginning on page 619.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: β2-adrenergic receptor (β2AR); protein kinase A (PKA); phospholipase C-β (PLCβ); inositol 1,4,5 trisphosphate (IP3); extracellular signal–regulated kinase (ERK); G protein receptor kinase (GRK).
References
- 1.Vathenen AS, Knox AJ, Higgins BG, Britton JR, Tattersfield AE. Rebound increase in bronchial responsiveness after treatment with inhaled terbutaline. Lancet. 1988;1:554–558. doi: 10.1016/s0140-6736(88)91352-9. [DOI] [PubMed] [Google Scholar]
- 2.Israel E, et al. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am. J. Respir. Crit. Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- 3.Taylor DR, et al. Asthma exacerbations during long term beta agonist use: influence of beta(2) adrenoceptor polymorphism. Thorax. 2000;55:762–767. doi: 10.1136/thorax.55.9.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor DR, et al. Asthma control during long-term treatment with regular inhaled salbutamol and salmeterol. Thorax. 1998;53:744–752. doi: 10.1136/thx.53.9.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hancox RJ, Sears MR, Taylor DR. Polymorphism of the beta2-adrenoceptor and the response to long-term beta2-agonist therapy in asthma. Eur. Respir. J. 1998;11:589–593. [PubMed] [Google Scholar]
- 6.Sears MR, et al. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet. 1990;336:1391–1396. doi: 10.1016/0140-6736(90)93098-a. [DOI] [PubMed] [Google Scholar]
- 7.Hanania NA, Sharafkhaneh A, Barber R, Dickey BF. Beta-agonist intrinsic efficacy: measurement and clinical significance. Am. J. Respir. Crit. Care Med. 2002;165:1353–1358. doi: 10.1164/rccm.2109060. [DOI] [PubMed] [Google Scholar]
- 8.McGraw DW, Almoosa KF, Paul RJ, Kobilka BK, Liggett SB. Antithetic regulation by β-adrenergic receptors of Gq receptor signaling via phospholipase C underlies the airway β-agonist paradox. J. Clin. Invest. 2003;112:619–626. doi:10.1172/JCI200318193. doi: 10.1172/JCI18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tohgo A, et al. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J. Biol. Chem. 2003;278:6258–6267. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- 10.Crespo P, Cachero TG, Xu N, Gutkind JS. Dual effect of beta-adrenergic receptors on mitogen-activated protein kinase. Evidence for a beta gamma-dependent activation and a G alpha s-cAMP-mediated inhibition. J. Biol. Chem. 1995;270:25259–25265. doi: 10.1074/jbc.270.42.25259. [DOI] [PubMed] [Google Scholar]
- 11.Zhu WZ, et al. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1607–1612. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou Y, et al. Both Gs and Gi proteins are critically involved in isoproterenol-induced cardiomyocyte hypertrophy. J. Biol. Chem. 1999;274:9760–9770. doi: 10.1074/jbc.274.14.9760. [DOI] [PubMed] [Google Scholar]
- 13.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 14.Hall RA, Lefkowitz RJ. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ. Res. 2002;91:672–680. doi: 10.1161/01.res.0000037000.74258.03. [DOI] [PubMed] [Google Scholar]
- 15.Cook SJ, McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993;262:1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- 16.Orsini MJ, et al. MAPK superfamily activation in human airway smooth muscle: mitogenesis requires prolonged p42/p44 activation. Am. J. Physiol. 1999;277:L479–L488. doi: 10.1152/ajplung.1999.277.3.L479. [DOI] [PubMed] [Google Scholar]
- 17.Hallsworth MP, Moir LM, Lai D, Hirst SJ. Inhibitors of mitogen-activated protein kinases differentially regulate eosinophil-activating cytokine release from human airway smooth muscle. Am. J. Respir. Crit. Care Med. 2001;164:688–697. doi: 10.1164/ajrccm.164.4.2011004. [DOI] [PubMed] [Google Scholar]
- 18.Roth M, et al. Interaction between glucocorticoids and beta2 agonists on bronchial airway smooth muscle cells through synchronised cellular signalling. Lancet. 2002;360:1293–1299. doi: 10.1016/S0140-6736(02)11319-5. [DOI] [PubMed] [Google Scholar]
- 19.Pang L, Knox AJ. Regulation of TNF-alpha-induced eotaxin release from cultured human airway smooth muscle cells by beta2-agonists and corticosteroids. FASEB J. 2001;15:261–269. doi: 10.1096/fj.00-0103com. [DOI] [PubMed] [Google Scholar]
- 20.Mak JC, Hisada T, Salmon M, Barnes PJ, Chung KF. Glucocorticoids reverse IL-1beta-induced impairment of beta-adrenoceptor-mediated relaxation and up-regulation of G-protein-coupled receptor kinases. Br. J. Pharmacol. 2002;135:987–996. doi: 10.1038/sj.bjp.0704545. [DOI] [PMC free article] [PubMed] [Google Scholar]