Abstract
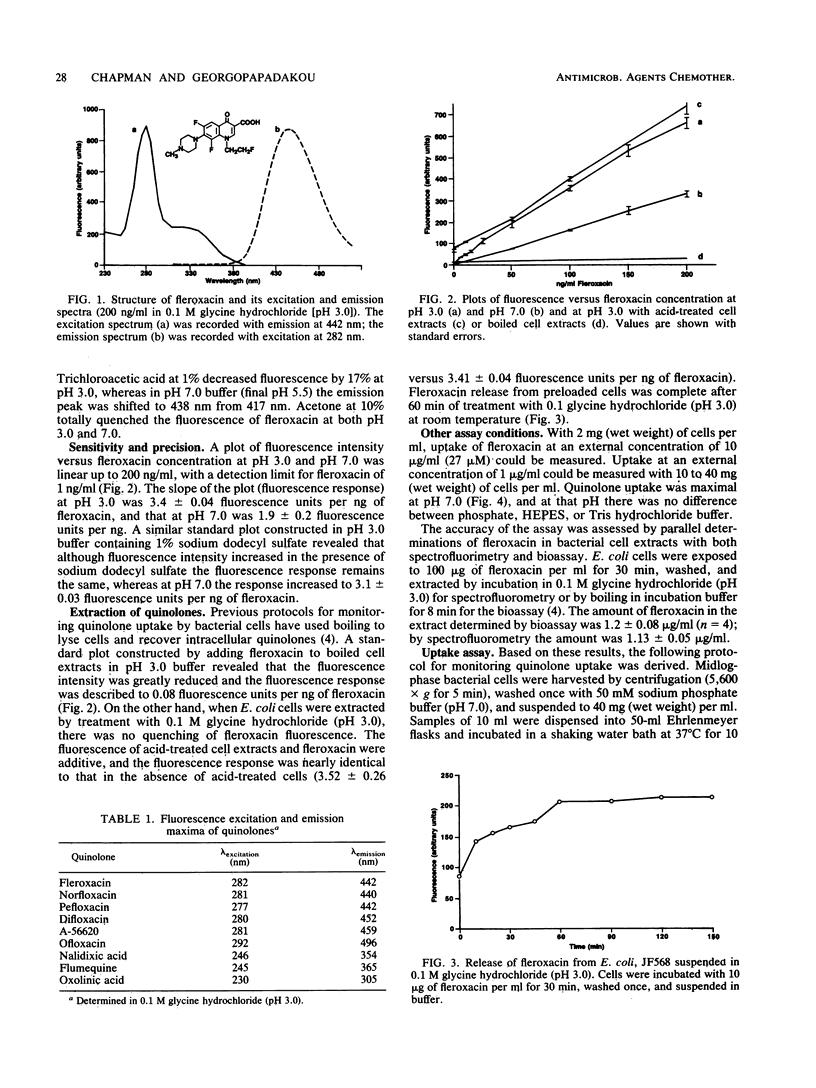
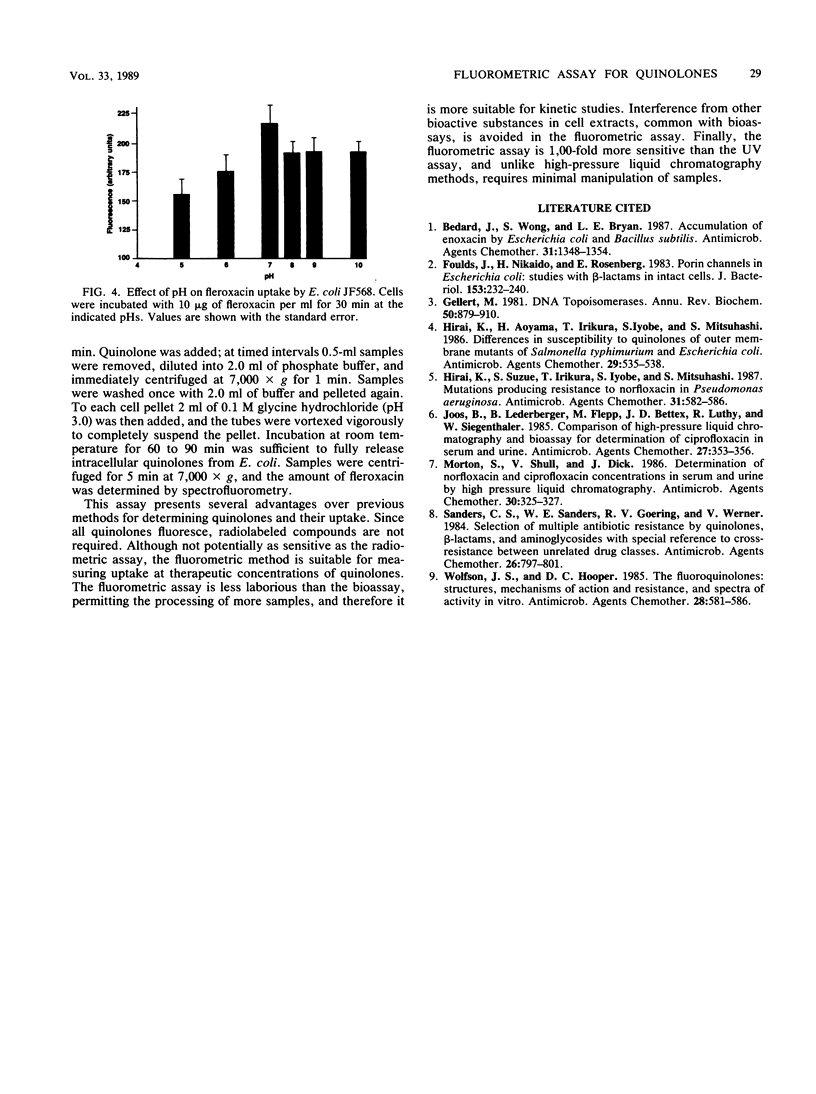
A sensitive and convenient method for quinolone determination has been developed, based on the natural fluorescence of the quinolone nucleus. Fleroxacin (Ro 23-6240; AM 833), used as a prototype quinolone in these studies, had an excitation maximum at 282 nm and an admission maximum at 442 nm (pH 3.0). Fluorescence intensity was pH dependent, being maximal at pH 3.0 and linear at quinolone concentrations between 1 and 200 ng/ml. A protocol for the fluorometric monitoring of fleroxacin uptake in Escherichia coli was developed. Intracellular quinolone concentrations measured by the fluorometric assay correlated well with values obtained by the bioassay. The results indicate that the fluorometric assay is an attractive alternative to the more laborious bioassay.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedard J., Wong S., Bryan L. E. Accumulation of enoxacin by Escherichia coli and Bacillus subtilis. Antimicrob Agents Chemother. 1987 Sep;31(9):1348–1354. doi: 10.1128/aac.31.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Apr;31(4):582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos B., Ledergerber B., Flepp M., Bettex J. D., Lüthy R., Siegenthaler W. Comparison of high-pressure liquid chromatography and bioassay for determination of ciprofloxacin in serum and urine. Antimicrob Agents Chemother. 1985 Mar;27(3):353–356. doi: 10.1128/aac.27.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton S. J., Shull V. H., Dick J. D. Determination of norfloxacin and ciprofloxacin concentrations in serum and urine by high-pressure liquid chromatography. Antimicrob Agents Chemother. 1986 Aug;30(2):325–327. doi: 10.1128/aac.30.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]



