Abstract
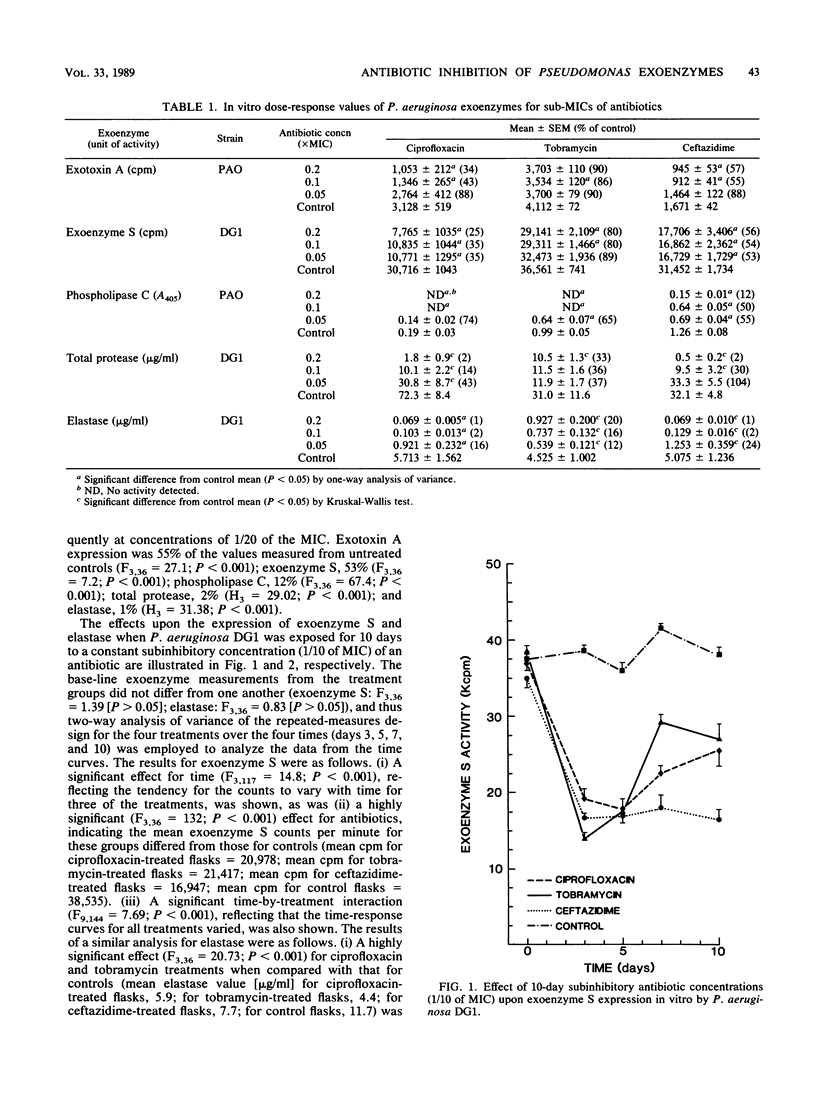
We examined the effects of subinhibitory concentrations of ciprofloxacin, tobramycin, and ceftazidime on Pseudomonas aeruginosa exoenzyme expression in vitro and in vivo. Exotoxin A, exoenzyme S, phospholipase C, elastase, and total protease activities were suppressed by antibiotics at concentrations as low as 1/20 of the MIC over a 24-h period in broth. Continuous 10-day exposure of P. aeruginosa DG1 broth cultures to antibiotic levels equal to 1/10 of the MIC reduced exoenzyme S activity in all treatment groups. Elastase activity was reduced only by ciprofloxacin and tobramycin treatment. This suppressive effect of the antibiotics persisted throughout the 10 days and was not influenced by the increase in MIC of ciprofloxacin detected during the course of the experiment. Rats chronically infected with P. aeruginosa were treated with subinhibitory doses of antibiotics and compared with untreated controls. Bacterial numbers in lung homogenates from each of the four study groups were identical. However, the lungs from antibiotic-treated rats had significantly less histological damage than those from control rats (P less than 0.001). The protective effect was greatest for ciprofloxacin and tobramycin. Further, P. aeruginosa isolates from ciprofloxacin- and tobramycin-treated rats demonstrated significantly less exoenzyme S and elastase activity than isolates from untreated rats (P less than 0.001). Isolates from ceftazidime-treated lungs expressed less exoenzyme S activity (P less than 0.001) but an equivalent amount of elastase activity as isolates from controls. The suppression of P. aeruginosa exoenzymes may arrest progressive lung injury during chronic P. aeruginosa lung infections.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., LEIDY G. Determination of inherited traits of H. influenzae by desoxyribonucleic acid fractions isolated from type-specific cells. J Exp Med. 1951 Apr 1;93(4):345–359. doi: 10.1084/jem.93.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berka R. M., Gray G. L., Vasil M. L. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect Immun. 1981 Dec;34(3):1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Sokol P. A., Iglewski B. H. Influence of iron on yields of extracellular products in Pseudomonas aeruginosa cultures. J Bacteriol. 1979 Apr;138(1):193–200. doi: 10.1128/jb.138.1.193-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosso J. A., Black P. G., Matsen J. M. Ciprofloxacin versus tobramycin plus azlocillin in pulmonary exacerbations in adult patients with cystic fibrosis. Am J Med. 1987 Apr 27;82(4A):180–184. [PubMed] [Google Scholar]
- Cash H. A., Woods D. E., McCullough B., Johanson W. G., Jr, Bass J. A. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979 Mar;119(3):453–459. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- Geers T. A., Baker N. R. The effect of sublethal concentrations of aminoglycosides on adherence of Pseudomonas aeruginosa to hamster tracheal epithelium. J Antimicrob Chemother. 1987 May;19(5):561–568. doi: 10.1093/jac/19.5.561. [DOI] [PubMed] [Google Scholar]
- Geers T. A., Baker N. R. The effect of sublethal levels of antibiotics on the pathogenicity of Pseudomonas aeruginosa for tracheal tissue. J Antimicrob Chemother. 1987 May;19(5):569–578. doi: 10.1093/jac/19.5.569. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M., Miniter P., Andriole V. T. Comparative efficacy of ciprofloxacin, azlocillin, and tobramycin alone and in combination in experimental Pseudomonas sepsis. J Infect Dis. 1987 Apr;155(4):783–788. doi: 10.1093/infdis/155.4.783. [DOI] [PubMed] [Google Scholar]
- MORIHARA K. PRODUCTION OF ELASTASE AND PROTEINASE BY PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Sep;88:745–757. doi: 10.1128/jb.88.3.745-757.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky I., McLaughlin F. J., Levison H. Cystic fibrosis: Part 1. Curr Probl Pediatr. 1985 Jun;15(6):1–49. doi: 10.1016/0045-9380(85)90061-1. [DOI] [PubMed] [Google Scholar]
- Marks M. I. The pathogenesis and treatment of pulmonary infections in patients with cystic fibrosis. J Pediatr. 1981 Feb;98(2):173–179. doi: 10.1016/s0022-3476(81)80631-2. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Ciprofloxacin: an overview and prospective appraisal. Am J Med. 1987 Apr 27;82(4A):395–404. [PubMed] [Google Scholar]
- Nicas T. I., Frank D. W., Stenzel P., Lile J. D., Iglewski B. H. Role of exoenzyme S in chronic Pseudomonas aeruginosa lung infections. Eur J Clin Microbiol. 1985 Apr;4(2):175–179. doi: 10.1007/BF02013593. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Iglewski B. H. The contribution of exoproducts to virulence of Pseudomonas aeruginosa. Can J Microbiol. 1985 Apr;31(4):387–392. doi: 10.1139/m85-074. [DOI] [PubMed] [Google Scholar]
- Pitcher-Wilmott R. W., Levinsky R. J., Gordon I., Turner M. W., Matthew D. J. Pseudomonas infection, allergy, and cystic fibrosis. Arch Dis Child. 1982 Aug;57(8):582–586. doi: 10.1136/adc.57.8.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S. H., Thompson W. L., Luthe M. A., Stern R. C., Grossniklaus D. A., Bloxham D. D., Groden D. L., Jacobs M. R., DiScenna A. O., Cash H. A. Once-daily vs. continuous aminoglycoside dosing: efficacy and toxicity in animal and clinical studies of gentamicin, netilmicin, and tobramycin. J Infect Dis. 1983 May;147(5):918–932. doi: 10.1093/infdis/147.5.918. [DOI] [PubMed] [Google Scholar]
- Que J. U., Woods D. E. Alteration of lung structure and function by Pseudomonas aeruginosa. Pathol Immunopathol Res. 1987;6(2):93–102. doi: 10.1159/000157051. [DOI] [PubMed] [Google Scholar]
- Roosendaal R., Bakker-Woudenberg I. A., van den Berghe-van Raffe M., Vink-van den Berg J. C., Michel M. F. Comparative activities of ciprofloxacin and ceftazidime against Klebsiella pneumoniae in vitro and in experimental pneumonia in leukopenic rats. Antimicrob Agents Chemother. 1987 Nov;31(11):1809–1815. doi: 10.1128/aac.31.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaad U. B., Wedgwood-Krucko J., Suter S., Kraemer R. Efficacy of inhaled amikacin as adjunct to intravenous combination therapy (ceftazidime and amikacin) in cystic fibrosis. J Pediatr. 1987 Oct;111(4):599–605. doi: 10.1016/s0022-3476(87)80130-0. [DOI] [PubMed] [Google Scholar]
- Scully B. E., Neu H. C., Parry M. F., Mandell W. Oral ciprofloxacin therapy of infections due to Pseudomonas aeruginosa. Lancet. 1986 Apr 12;1(8485):819–822. doi: 10.1016/s0140-6736(86)90937-2. [DOI] [PubMed] [Google Scholar]
- Shibl A. M., Al-Sowaygh I. A. Antibiotic inhibition of protease production by Pseudomonas aeruginosa. J Med Microbiol. 1980 May;13(2):345–348. doi: 10.1099/00222615-13-2-345. [DOI] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Relationship of iron and extracellular virulence factors to Pseudomonas aeruginosa lung infections. J Med Microbiol. 1984 Aug;18(1):125–133. doi: 10.1099/00222615-18-1-125. [DOI] [PubMed] [Google Scholar]
- Vishwanath S., Guay C. M., Ramphal R. Effects of subminimal inhibitory concentrations of antibiotics on the adherence of Pseudomonas aeruginosa to tracheobronchial mucin. J Antimicrob Chemother. 1987 May;19(5):579–583. doi: 10.1093/jac/19.5.579. [DOI] [PubMed] [Google Scholar]
- Warren R. L., Baker N. R., Johnson J., Stapleton M. J. Selective inhibition of the accumulation of extracellular proteases of Pseudomonas aeruginosa by gentamicin and tobramycin. Antimicrob Agents Chemother. 1985 Apr;27(4):468–472. doi: 10.1128/aac.27.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. D., Moosdeen F. In vitro antibacterial effects of cephalosporins. Drugs. 1987;34 (Suppl 2):44–63. doi: 10.2165/00003495-198700342-00006. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Cryz S. J., Friedman R. L., Iglewski B. H. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect Immun. 1982 Jun;36(3):1223–1228. doi: 10.1128/iai.36.3.1223-1228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Que J. U. Purification of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1987 Mar;55(3):579–586. doi: 10.1128/iai.55.3.579-586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Schaffer M. S., Rabin H. R., Campbell G. D., Sokol P. A. Phenotypic comparison of Pseudomonas aeruginosa strains isolated from a variety of clinical sites. J Clin Microbiol. 1986 Aug;24(2):260–264. doi: 10.1128/jcm.24.2.260-264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Sokol P. A. Role of Pseudomonas aeruginosa extracellular enzymes in lung disease. Clin Invest Med. 1986;9(2):108–112. [PubMed] [Google Scholar]
- Woods D. E., Sokol P. A. Use of transposon mutants to assess the role of exoenzyme S in chronic pulmonary disease due to Pseudomonas aeruginosa. Eur J Clin Microbiol. 1985 Apr;4(2):163–169. doi: 10.1007/BF02013591. [DOI] [PubMed] [Google Scholar]



