Abstract
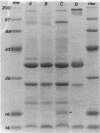
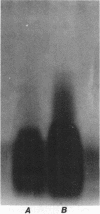
The mechanism of chloramphenicol resistance was examined in a high-level-resistant isolate of Pseudomonas cepacia from a patient with cystic fibrosis. We investigated potential resistance mechanisms, including production of chloramphenicol acetyltransferase, ribosomal resistance, and decreased permeability. This strain (MIC, 200 micrograms/ml) had no detectable chloramphenicol acetyltransferase activity. In in vitro translation experiments in which we compared the resistant isolate with a susceptible strain of P. cepacia, inhibition of amino acid incorporation was equivalent even in organisms that were preincubated with sub-MICs of chloramphenicol. A 21.9-kilobase (kb) fragment of DNA was cloned which coded for chloramphenicol resistance; this fragment was expressed in P. cepacia but not in Escherichia coli. Quantitation of chloramphenicol uptake in the isogenic pair of susceptible and resistant organisms revealed a nearly 10-fold decrease of drug entry into the resistant strain. Comparison of isolated outer membrane proteins and lipopolysaccharide patterns identified no significant differences between the isogenic pair of organisms. We concluded that the mechanism of chloramphenicol resistance in this strain is decreased permeability.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman W., Lessie T. G. Response of Pseudomonas cepacia to beta-Lactam antibiotics: utilization of penicillin G as the carbon source. J Bacteriol. 1979 Dec;140(3):1126–1128. doi: 10.1128/jb.140.3.1126-1128.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bosso J. A., Saxon B. A., Matsen J. M. In vitro activity of aztreonam combined with tobramycin and gentamicin against clinical isolates of Pseudomonas aeruginosa and Pseudomonas cepacia from patients with cystic fibrosis. Antimicrob Agents Chemother. 1987 Sep;31(9):1403–1405. doi: 10.1128/aac.31.9.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Mendelman P. M., Levy J., Stull T. L., Smith A. L. A permeability barrier as a mechanism of chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1985 Jan;27(1):46–54. doi: 10.1128/aac.27.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Rubens C. E., Mendelman P. M., Smith A. L. Cloning and expression in Escherichia coli of a gene encoding nonenzymatic chloramphenicol resistance from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1986 Mar;29(3):445–450. doi: 10.1128/aac.29.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Smith A. L. Chloramphenicol accumulation by Haemophilus influenzae. Antimicrob Agents Chemother. 1987 May;31(5):686–690. doi: 10.1128/aac.31.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa C., Labrozzi P. H., Aronoff S. C. Decreased baseline beta-lactamase production and inducibility associated with increased piperacillin susceptibility of Pseudomonas cepacia isolated from children with cystic fibrosis. Pediatr Res. 1986 Nov;20(11):1174–1177. doi: 10.1203/00006450-198611000-00026. [DOI] [PubMed] [Google Scholar]
- Cozens R. M., Brown M. R. Effect of nutrient depletion on the sensitivity of Pseudomonas cepacia to antimicrobial agents. J Pharm Sci. 1983 Nov;72(11):1363–1365. doi: 10.1002/jps.2600721135. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ederer G. M., Matsen J. M. Colonization and infection with Pseudomonas cepacia. J Infect Dis. 1972 Jun;125(6):613–618. doi: 10.1093/infdis/125.6.613. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney D. F., Cundliffe E., Foster T. J. Chloramphenicol resistance that does not involve chloramphenicol acetyltransferase encoded by plasmids from gram-negative bacteria. J Gen Microbiol. 1981 Jul;125(1):113–121. doi: 10.1099/00221287-125-1-113. [DOI] [PubMed] [Google Scholar]
- Gold R., Jin E., Levison H., Isles A., Fleming P. C. Ceftazidime alone and in combination in patients with cystic fibrosis: lack of efficacy in treatment of severe respiratory infections caused by Pseudomonas cepacia. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):331–336. doi: 10.1093/jac/12.suppl_a.331. [DOI] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J Bacteriol. 1984 Jun;158(3):1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- Hardy P. C., Ederer G. M., Matsen J. M. Contamination of commercially packaged urinary catheter kits with the pseudomonad EO-1. N Engl J Med. 1970 Jan 1;282(1):33–35. doi: 10.1056/NEJM197001012820108. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Isles A., Maclusky I., Corey M., Gold R., Prober C., Fleming P., Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984 Feb;104(2):206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- Kaslow R. A., Mackel D. C., Mallison G. F. Nosocomial pseudobacteremia. Positive blood cultures due to contaminated benzalkonium antiseptic. JAMA. 1976 Nov 22;236(21):2407–2409. doi: 10.1001/jama.236.21.2407. [DOI] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Kono M., O'Hara K. Mechanism of chloramphenicol-resistance mediated by kR102 factor in Pseudomonas aeruginosa. J Antibiot (Tokyo) 1976 Feb;29(2):176–180. doi: 10.7164/antibiotics.29.176. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martone W. J., Osterman C. A., Fisher K. A., Wenzel R. P. Pseudomonas cepacia: implications and control of epidemic nosocomial colonization. Rev Infect Dis. 1981 Jul-Aug;3(4):708–715. doi: 10.1093/clinids/3.4.708. [DOI] [PubMed] [Google Scholar]
- Moore R. A., Hancock R. E. Involvement of outer membrane of Pseudomonas cepacia in aminoglycoside and polymyxin resistance. Antimicrob Agents Chemother. 1986 Dec;30(6):923–926. doi: 10.1128/aac.30.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. A., Close J. A. Edetate disodium-mediated chloramphenicol resistance in Pseudomonas cepacia. J Pharm Sci. 1982 Jul;71(7):833–834. doi: 10.1002/jps.2600710732. [DOI] [PubMed] [Google Scholar]
- Osawa S., Takata R., Tanaka K., Tamaki M. Chloramphenicol resistant mutants of Bacillus subtilis. Mol Gen Genet. 1973 Dec 20;127(2):163–173. doi: 10.1007/BF00333664. [DOI] [PubMed] [Google Scholar]
- Parr T. R., Jr, Moore R. A., Moore L. V., Hancock R. E. Role of porins in intrinsic antibiotic resistance of Pseudomonas cepacia. Antimicrob Agents Chemother. 1987 Jan;31(1):121–123. doi: 10.1128/aac.31.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin R. H. Pseudomonas cepacia in an intensive care nursery. Pediatrics. 1976 Feb;57(2):239–243. [PubMed] [Google Scholar]
- Richards R. M., Richards J. M. Pseudomonas cepacia resistance to antibacterials. J Pharm Sci. 1979 Nov;68(11):1436–1438. doi: 10.1002/jps.2600681127. [DOI] [PubMed] [Google Scholar]
- Rosenstein B. J., Hall D. E. Pneumonia and septicemia due to Pseudomonas cepacia in a patient with cystic fibrosis. Johns Hopkins Med J. 1980 Nov;147(5):188–189. [PubMed] [Google Scholar]
- Schmidhauser T. J., Helinski D. R. Regions of broad-host-range plasmid RK2 involved in replication and stable maintenance in nine species of gram-negative bacteria. J Bacteriol. 1985 Oct;164(1):446–455. doi: 10.1128/jb.164.1.446-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scordilis G. E., Ree H., Lessie T. G. Identification of transposable elements which activate gene expression in Pseudomonas cepacia. J Bacteriol. 1987 Jan;169(1):8–13. doi: 10.1128/jb.169.1.8-13.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Stull T. L., Mack K., Haas J. E., Smit J., Smith A. L. A comparison of techniques for isolation of the outer membrane proteins of Haemophilus influenzae type b. Anal Biochem. 1985 Nov 1;150(2):471–480. doi: 10.1016/0003-2697(85)90537-8. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Welch D. F., Muszynski M. J., Pai C. H., Marcon M. J., Hribar M. M., Gilligan P. H., Matsen J. M., Ahlin P. A., Hilman B. C., Chartrand S. A. Selective and differential medium for recovery of Pseudomonas cepacia from the respiratory tracts of patients with cystic fibrosis. J Clin Microbiol. 1987 Sep;25(9):1730–1734. doi: 10.1128/jcm.25.9.1730-1734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]