Abstract
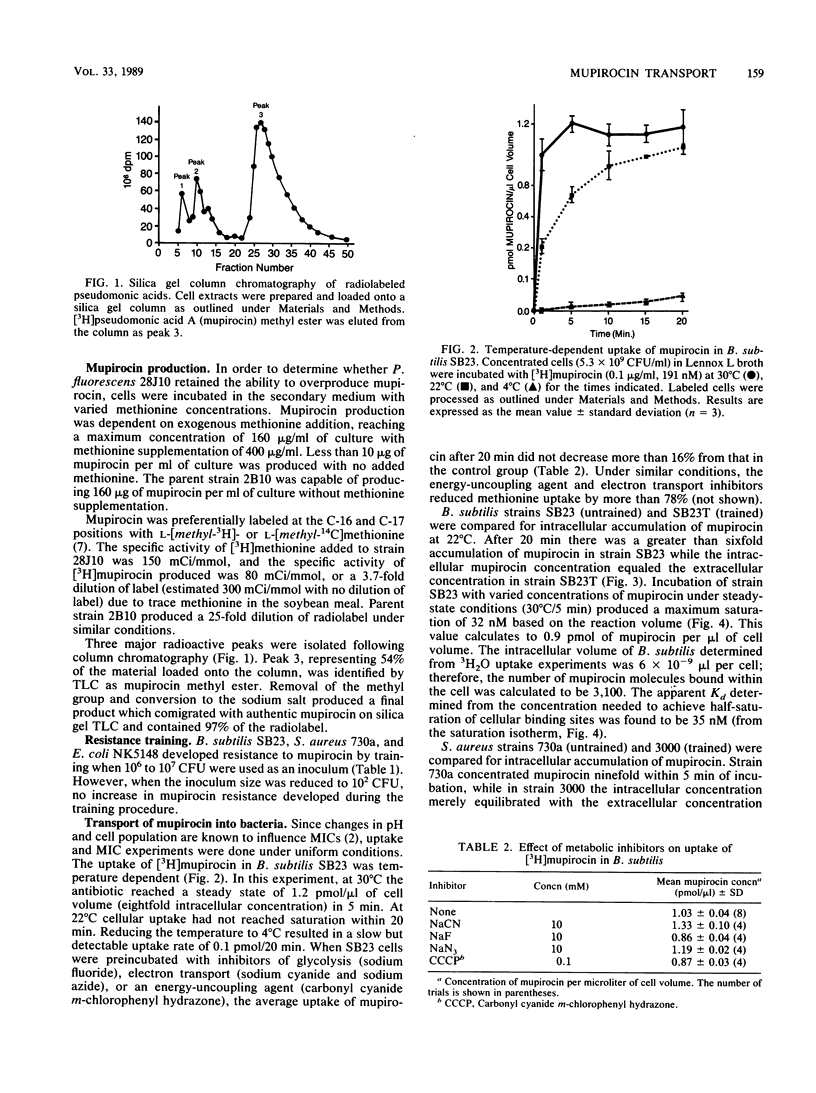
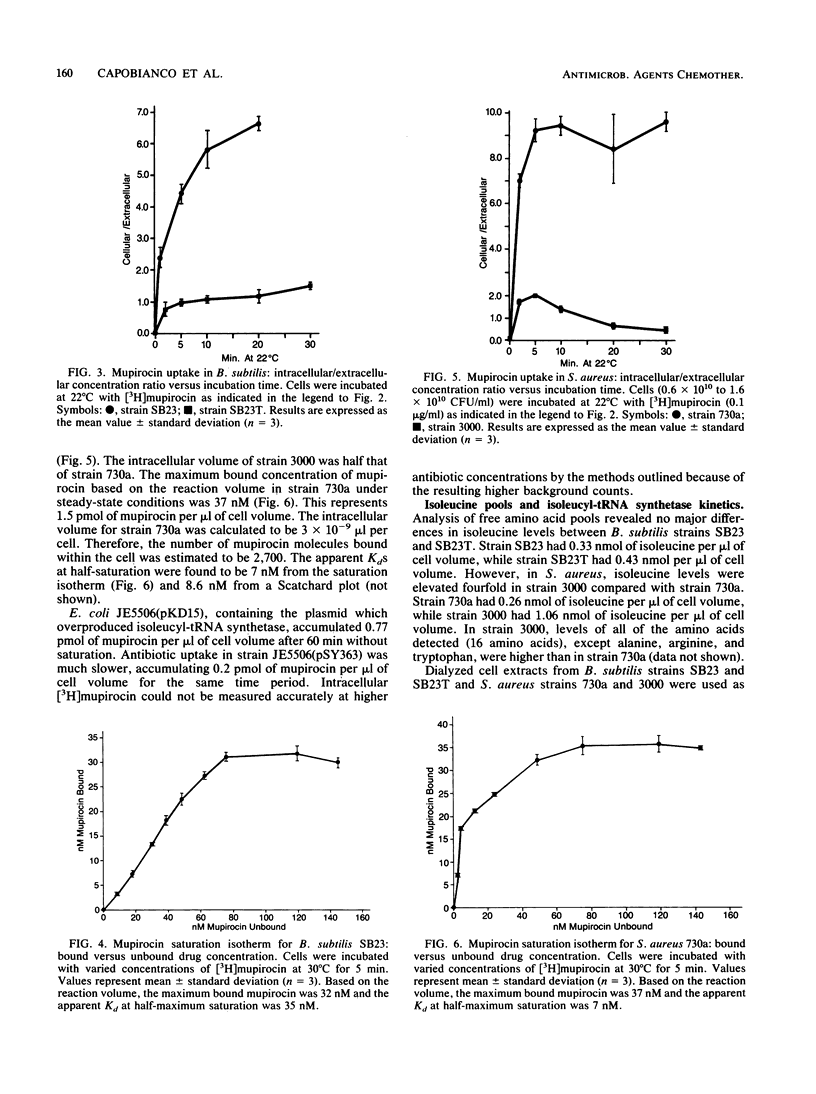
Pseudomonic acid A (mupirocin) blocks protein synthesis in bacteria by inhibition of bacterial isoleucyl-tRNA synthetase. [16, 17-3H]mupirocin, isolated from a methionine auxotroph of Pseudomonas fluorescens, was used to study transport of this antibiotic into sensitive and resistant strains of Bacillus subtilis, Staphylococcus aureus, and Escherichia coli. The transport of mupirocin into sensitive bacteria was energy independent and temperature dependent (decreased uptake at lower temperatures), indicating non-carrier-mediated passive diffusion. Uptake was also saturable with time or increasing antibiotic concentration. The saturable intracellular binding site, most likely the target isoleucyl-tRNA synthetase as determined by the amount of bound mupirocin (2,700 to 3,100 molecules per cell), caused concentration of the antibiotic within the cell. E. coli transformed with a plasmid containing ileS overproduced the target enzyme and demonstrated greater accumulation of mupirocin than a strain containing a control plasmid. The concentrations needed to half saturate (Kd) these binding sites in B. subtilis and S. aureus were 35 and 7 nM, respectively. In gram-positive organisms trained for mupirocin resistance, uptake was not saturable with increasing antibiotic concentration, and intra- and extracellular concentrations of drug equilibrated with time. Kinetic analysis of crude isoleucyl-tRNA synthetase from trained and untrained B. subtilis strains revealed differences in apparent Ki for mupirocin (resistant strain SB23T, Ki = 71.1 nM; sensitive strain SB23, Ki = 33.5 nM), while the Km for isoleucine remained unchanged (2.7 to 2.9 microM). A Km of 0.4 micromolar isoleucine and Ki of 24 nM mupirocin was demonstrated for isoleucyl-tRNA synthetase from sensitive S. aureus 730a, while no isoleucyl-tRNA synthetase activity was detected in extracts of resistance-trained S. aureus 3000 even at 40 micromolar isoleucine, suggesting instability of the enzyme. Free isoleucine pools differed between sensitive (0.26 micromolar) and resistance-trained (1.06 micromolar) S. aureus. Our results demonstrate that (i) mupirocin enters cells by passive diffusion, (ii) mupirocin concentrates in sensitive bacteria due to binding to isoleucyl-tRNA synthetase, and (iii) resistance to mupirocin involves restricted access to the binding site of isoleucyl-tRNA synthetase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calhoun D. H., Feary T. W. Transductional analysis of Pseudomonas aeruginosa methionineless auxotrophs. J Bacteriol. 1969 Jan;97(1):210–216. doi: 10.1128/jb.97.1.210-216.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casewell M. W., Hill R. L. In-vitro activity of mupirocin ('pseudomonic acid') against clinical isolates of Staphylococcus aureus. J Antimicrob Chemother. 1985 May;15(5):523–531. doi: 10.1093/jac/15.5.523. [DOI] [PubMed] [Google Scholar]
- Chain E. B., Mellows G. Pseudomonic acid. Part 1. The structure of pseudomonic acid A, a novel antibiotic produced by Pseudomonas fluorescens. J Chem Soc Perkin 1. 1977;(3):294–309. [PubMed] [Google Scholar]
- Chatterjee A. K., Sanderson K. E., Ross H. Influence of temperature on growth of lipopolysaccharide-deficient (rough) mutants of Salmonella typhimurium and Salmonella minnesota. Can J Microbiol. 1976 Oct;22(10):1540–1548. doi: 10.1139/m76-226. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Dureković A., Flossdorf J., Kula M. R. Isolation and properties of isoleucyl-tRNA synthetase from Escherichia coli MRE 600. Eur J Biochem. 1973 Jul 16;36(2):528–533. doi: 10.1111/j.1432-1033.1973.tb02939.x. [DOI] [PubMed] [Google Scholar]
- Feline T. C., Jones R. B., Mellows G., Phillips L. Pseudomonic acid. Part 2. Biosynthesis of pseudomonic acid A. J Chem Soc Perkin 1. 1977;(3):309–318. [PubMed] [Google Scholar]
- Fuller A. T., Mellows G., Woolford M., Banks G. T., Barrow K. D., Chain E. B. Pseudomonic acid: an antibiotic produced by Pseudomonas fluorescens. Nature. 1971 Dec 17;234(5329):416–417. doi: 10.1038/234416a0. [DOI] [PubMed] [Google Scholar]
- Harris C. L. An aminoacyl-tRNA synthetase complex in Escherichia coli. J Bacteriol. 1987 Jun;169(6):2718–2723. doi: 10.1128/jb.169.6.2718-2723.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K. B., Lin E. C., Wilson T. H. Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol. 1980 Oct;144(1):274–278. doi: 10.1128/jb.144.1.274-278.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Mellows G. Inhibition of isoleucyl-transfer ribonucleic acid synthetase in Escherichia coli by pseudomonic acid. Biochem J. 1978 Oct 15;176(1):305–318. doi: 10.1042/bj1760305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Mellows G. Interaction of pseudomonic acid A with Escherichia coli B isoleucyl-tRNA synthetase. Biochem J. 1980 Oct 1;191(1):209–219. doi: 10.1042/bj1910209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Mellows G. On the mode of action of pseudomonic acid: inhibition of protein synthesis in Staphylococcus aureus. J Antibiot (Tokyo) 1978 Apr;31(4):330–335. doi: 10.7164/antibiotics.31.330. [DOI] [PubMed] [Google Scholar]
- Hughes J., Mellows G., Soughton S. How does Pseudomonas fluorescens, the producing organism of the antibiotic pseudomonic acid A, avoid suicide? FEBS Lett. 1980 Dec 29;122(2):322–324. doi: 10.1016/0014-5793(80)80465-0. [DOI] [PubMed] [Google Scholar]
- Iaccarino M., Berg P. Isoleucine auxotrophy as a consequence of a mutationally altered isoleucyl-transfer ribonucleic acid synthetase. J Bacteriol. 1971 Feb;105(2):527–537. doi: 10.1128/jb.105.2.527-537.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Hand W. L., Francis J. B., King-Thompson N., Corwin R. W. Antibiotic uptake by alveolar macrophages. J Lab Clin Med. 1980 Mar;95(3):429–439. [PubMed] [Google Scholar]
- Kawakami M., Miyazaki M., Yamada H., Mizushima S. Isolation of gram quantities of isoleucyl-tRNA synthetase from an overproducing strain of Escherichia coli and its use for purification of cognate tRNA. FEBS Lett. 1985 Jun 3;185(1):162–164. doi: 10.1016/0014-5793(85)80762-6. [DOI] [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Träuble H. Phase transitions in cells, membranes, and lipids of Escherichia coli. Detection by fluorescent probes, light scattering, and dilatometry. Biochemistry. 1973 Jul 3;12(14):2625–2634. doi: 10.1021/bi00738a012. [DOI] [PubMed] [Google Scholar]
- Parenti M. A., Hatfield S. M., Leyden J. J. Mupirocin: a topical antibiotic with a unique structure and mechanism of action. Clin Pharm. 1987 Oct;6(10):761–770. [PubMed] [Google Scholar]
- Sutherland R., Boon R. J., Griffin K. E., Masters P. J., Slocombe B., White A. R. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother. 1985 Apr;27(4):495–498. doi: 10.1128/aac.27.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Overath P. The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim Biophys Acta. 1973 May 25;307(3):491–512. doi: 10.1016/0005-2736(73)90296-4. [DOI] [PubMed] [Google Scholar]
- Ward A., Campoli-Richards D. M. Mupirocin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1986 Nov;32(5):425–444. doi: 10.2165/00003495-198632050-00002. [DOI] [PubMed] [Google Scholar]


