Abstract
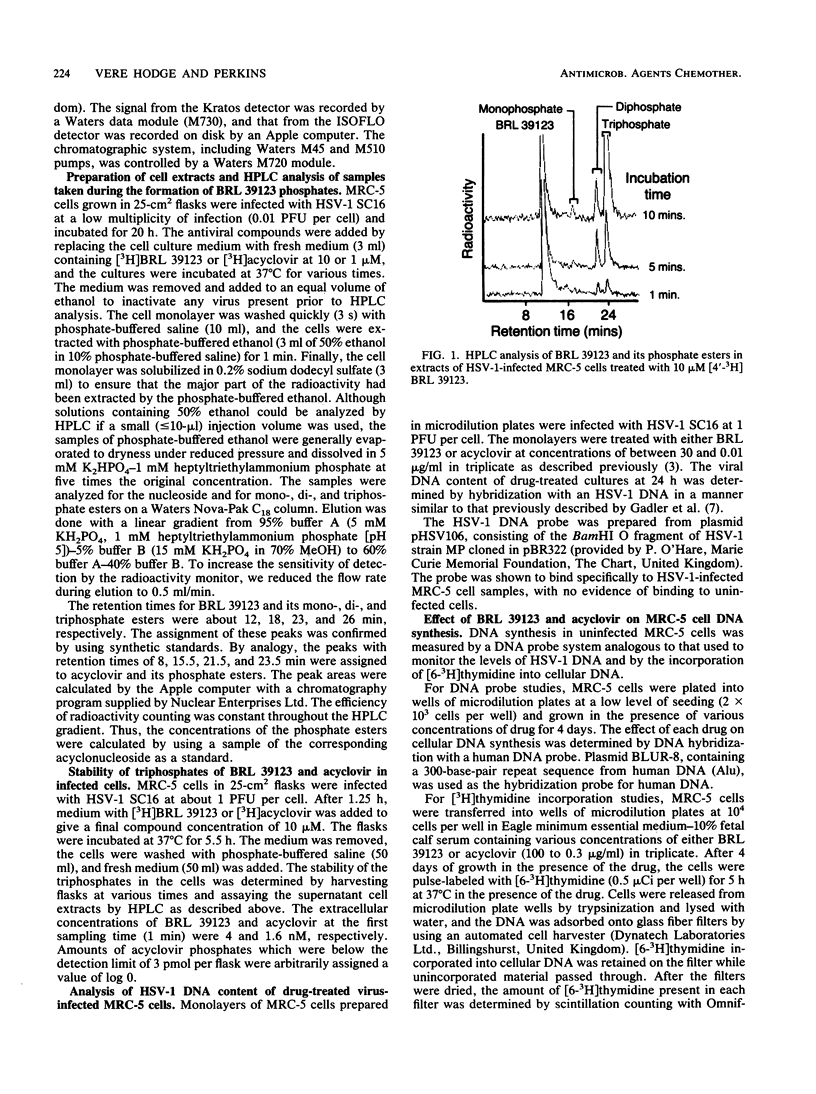
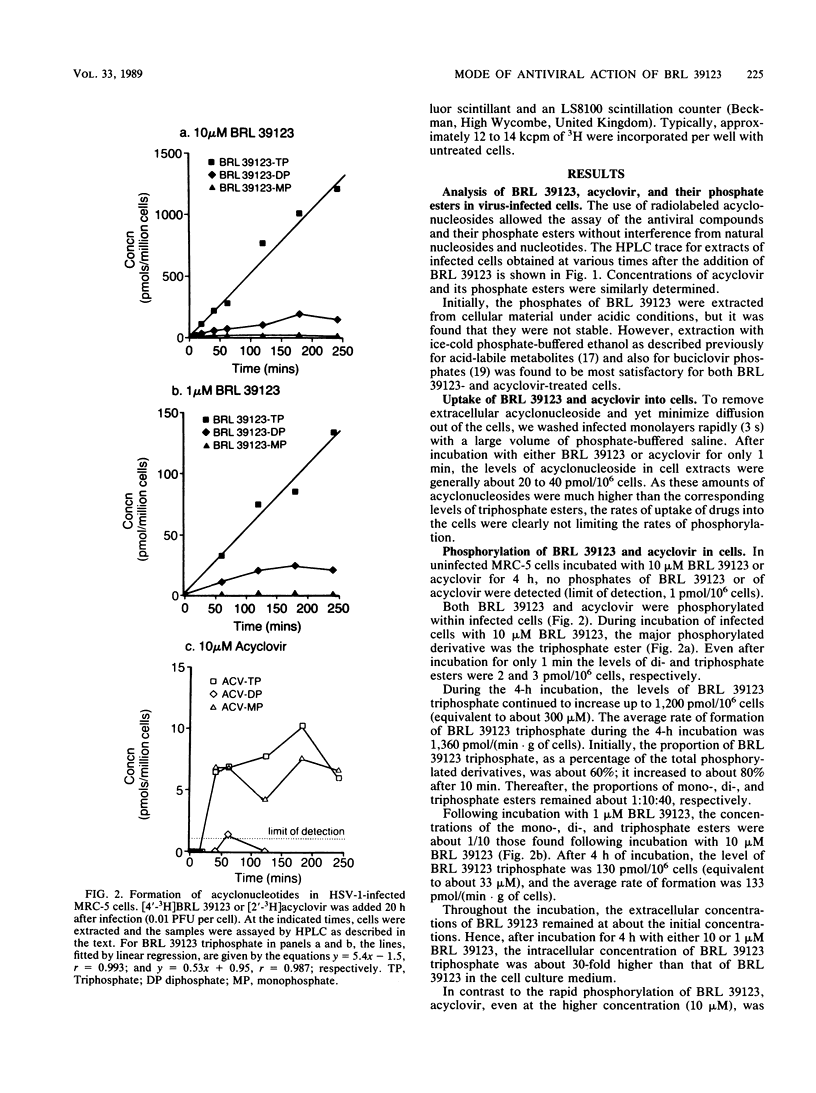
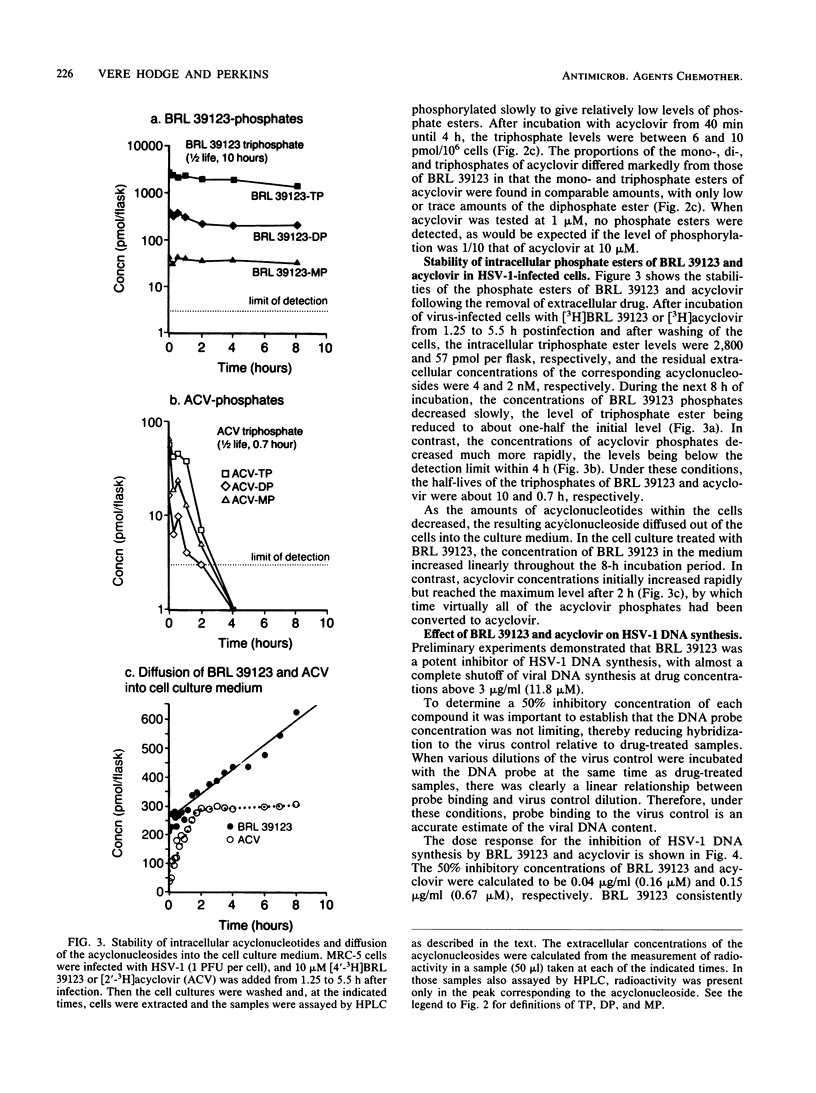
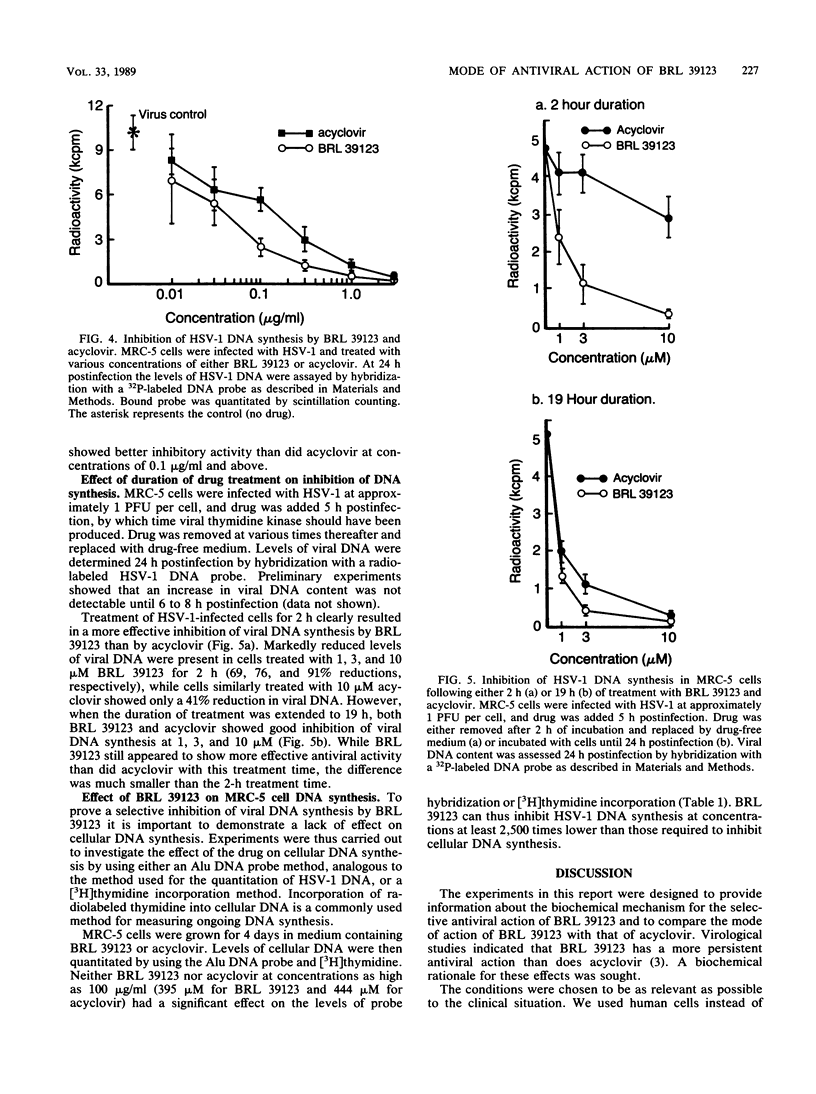
The metabolism and mode of action of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) were studied in herpes simplex virus type 1 (HSV-1)-infected and uninfected MRC-5 cells and compared with those of acyclovir. In uninfected cells incubated with 10 microM acyclonucleoside for 4 h, no phosphorylation of either BRL 39123 or acyclovir was detected. In contrast, in HSV-1-infected cells, both BRL 39123 and acyclovir were phosphorylated up to the triphosphate esters. Phosphorylation of BRL 39123 occurred much more rapidly and proceeded to a greater extent than did that of acyclovir. Furthermore, following the removal of acyclonucleoside from the culture medium, the intracellular triphosphate ester of BRL 39123 was much more stable than was that of acyclovir, the half-lives being about 10 and 0.7 h, respectively. BRL 39123 treatment effectively inhibited the formation of HSV-1 DNA in infected MRC-5 cells, 50% inhibitory concentrations of BRL 39123 and acyclovir being 0.04 microgram/ml (0.16 microM) and 0.15 microgram/ml (0.67 microM), respectively. In addition, BRL 39123 was shown to be more effective than acyclovir at inhibiting viral DNA synthesis following short treatment times, presumably reflecting the greater stability of BRL 39123 triphosphate. Neither BRL 39123 nor acyclovir inhibited cellular DNA synthesis in uninfected cells at concentrations of up to 100 micrograms/ml.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd M. R., Bacon T. H., Sutton D. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxymethylbut-1-yl) guanine (BRL 39123) in animals. Antimicrob Agents Chemother. 1988 Mar;32(3):358–363. doi: 10.1128/aac.32.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd M. R., Bacon T. H., Sutton D., Cole M. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxy-methylbut-1-yl)guanine (BRL 39123) in cell culture. Antimicrob Agents Chemother. 1987 Aug;31(8):1238–1242. doi: 10.1128/aac.31.8.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datema R., Ericson A. C., Field H. J., Larsson A., Stenberg K. Critical determinants of antiherpes efficacy of buciclovir and related acyclic guanosine analogs. Antiviral Res. 1987 Jul;7(6):303–316. doi: 10.1016/0166-3542(87)90013-1. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., de Miranda P., St Clair M. H., Elion G. B. Metabolism of acyclovir in virus-infected and uninfected cells. Antimicrob Agents Chemother. 1981 Oct;20(4):518–524. doi: 10.1128/aac.20.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadler H., Larsson A., Sølver E. Nucleic acid hybridization, a method to determine effects of antiviral compounds on herpes simplex virus type 1 DNA synthesis. Antiviral Res. 1984 Apr;4(1-2):63–70. doi: 10.1016/0166-3542(84)90026-3. [DOI] [PubMed] [Google Scholar]
- Gentry G. A., Allen G. P., Holton R., Nevins R. B., McGowan J. J., Veerisetty V. Thymine salvage, mitochondria, and the evolution of the herpesviruses. Intervirology. 1983;19(2):67–76. doi: 10.1159/000149340. [DOI] [PubMed] [Google Scholar]
- Harmenberg J., Abele G., Wahren B. Nucleoside pools of acyclovir-treated herpes simplex type 1 infected cells. Antiviral Res. 1985 Apr;5(2):75–81. doi: 10.1016/0166-3542(85)90033-6. [DOI] [PubMed] [Google Scholar]
- Harnden M. R., Jarvest R. L., Bacon T. H., Boyd M. R. Synthesis and antiviral activity of 9-[4-hydroxy-3-(hydroxymethyl)but-1-yl]purines. J Med Chem. 1987 Sep;30(9):1636–1642. doi: 10.1021/jm00392a020. [DOI] [PubMed] [Google Scholar]
- Hill T. J., Field H. J., Blyth W. A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975 Sep;28(3):341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- Karlsson A. H., Harmenberg J. G., Wahren B. E. Influence of acyclovir and bucyclovir on nucleotide pools in cells infected with herpes simplex virus type 1. Antimicrob Agents Chemother. 1986 May;29(5):821–824. doi: 10.1128/aac.29.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A., Stenberg K., Ericson A. C., Haglund U., Yisak W. A., Johansson N. G., Oberg B., Datema R. Mode of action, toxicity, pharmacokinetics, and efficacy of some new antiherpesvirus guanosine analogs related to buciclovir. Antimicrob Agents Chemother. 1986 Oct;30(4):598–605. doi: 10.1128/aac.30.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. H., Miller R. L. Phosphorylation of acyclovir diphosphate by cellular enzymes. Biochem Pharmacol. 1982 Dec 1;31(23):3879–3884. doi: 10.1016/0006-2952(82)90305-7. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Schwarz R. T., Scholtissek C. Nucleoside-diphosphate derivatives of 2-deoxy-D-glucose in animal cells. Eur J Biochem. 1974 Nov 1;49(1):237–247. doi: 10.1111/j.1432-1033.1974.tb03828.x. [DOI] [PubMed] [Google Scholar]
- Smee D. F., Boehme R., Chernow M., Binko B. P., Matthews T. R. Intracellular metabolism and enzymatic phosphorylation of 9-(1,3-dihydroxy-2-propoxymethyl)guanine and acyclovir in herpes simplex virus-infected and uninfected cells. Biochem Pharmacol. 1985 Apr 1;34(7):1049–1056. doi: 10.1016/0006-2952(85)90608-2. [DOI] [PubMed] [Google Scholar]
- Stenberg K., Larsson A., Datema R. Metabolism and mode of action of (R)-9-(3,4-dihydroxybutyl)guanine in herpes simplex virus-infected vero cells. J Biol Chem. 1986 Feb 15;261(5):2134–2139. [PubMed] [Google Scholar]


