Abstract
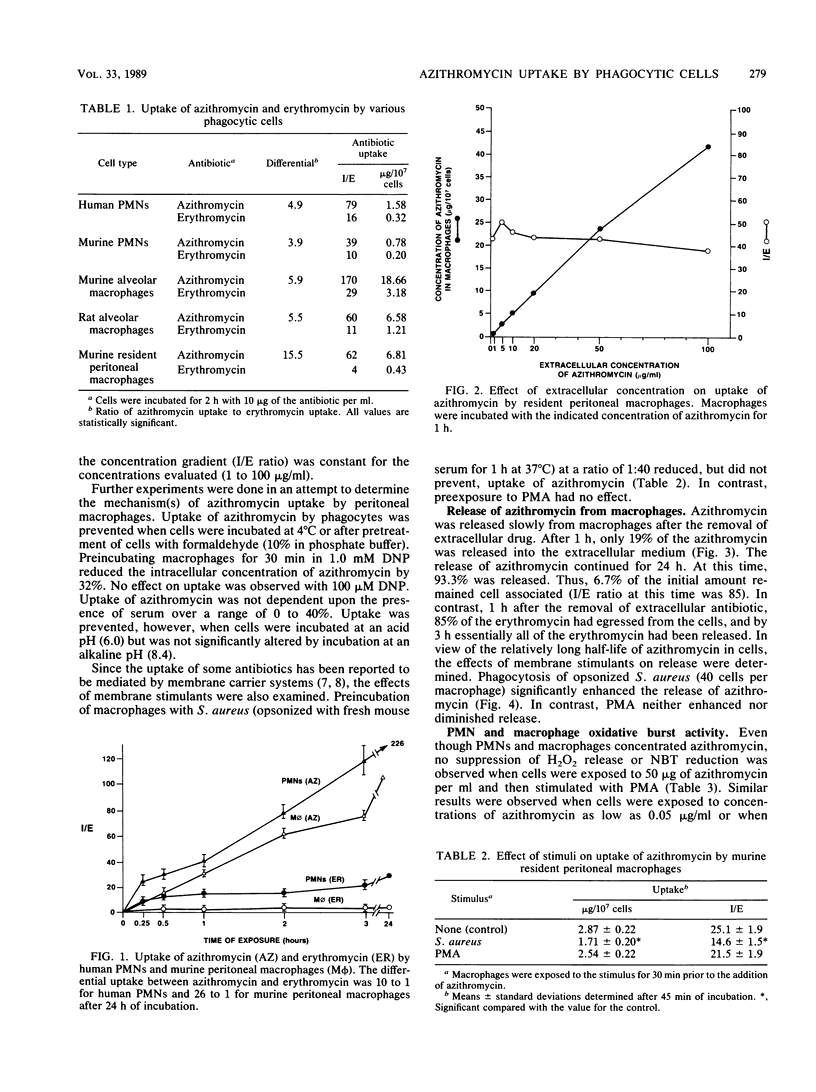
Azithromycin, a novel azalide antibiotic, concentrated in human and mouse polymorphonuclear leukocytes (PMNs), murine peritoneal macrophages, and mouse and rat alveolar macrophages, attaining intracellular concentrations up to 226 times the external concentration in vitro. In murine peritoneal macrophages, azithromycin achieved concentration gradients (internal to external) up to 26 times higher than erythromycin. The cellular uptake of azithromycin was dependent on temperature, viability, and pH and was decreased by 2,4-dinitrophenol. Azithromycin did not decrease phagocyte-mediated bactericidal activity or affect PMN or macrophage oxidative burst activity (H2O2 release or Nitro Blue Tetrazolium reduction, respectively). Azithromycin remained in cells for several hours, even after extracellular drug was removed. However, its release was significantly enhanced by phagocytosis of Staphylococcus aureus (82 versus 23% by 1.5 h). In vivo, 0.05 micrograms of azithromycin was found in peritoneal fluids of mice 20 h after oral treatment with a dose of 50 mg/kg. Following caseinate-induced PMN infiltration, there was a sixfold increase in peritoneal cavity azithromycin to 0.32 micrograms, most of which was intracellular. Therefore, the uptake, transport, and later release of azithromycin by these cells demonstrate that phagocytes may deliver active drug to sites of infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bright G. M., Nagel A. A., Bordner J., Desai K. A., Dibrino J. N., Nowakowska J., Vincent L., Watrous R. M., Sciavolino F. C., English A. R. Synthesis, in vitro and in vivo activity of novel 9-deoxo-9a-AZA-9a-homoerythromycin A derivatives; a new class of macrolide antibiotics, the azalides. J Antibiot (Tokyo) 1988 Aug;41(8):1029–1047. doi: 10.7164/antibiotics.41.1029. [DOI] [PubMed] [Google Scholar]
- Carlier M. B., Zenebergh A., Tulkens P. M. Cellular uptake and subcellular distribution of roxithromycin and erythromycin in phagocytic cells. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):47–56. doi: 10.1093/jac/20.suppl_b.47. [DOI] [PubMed] [Google Scholar]
- Deysine M., Chua A., Gerboth A. Selective delivery of antibiotics to experimental infection by autologous white blood cells. Surg Forum. 1979;30:38–39. [PubMed] [Google Scholar]
- Girard A. E., Girard D., English A. R., Gootz T. D., Cimochowski C. R., Faiella J. A., Haskell S. L., Retsema J. A. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987 Dec;31(12):1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladue R., Girard A., Newborg M. Enhanced antibacterial resistance in neutropenic mice treated with human recombinant interleukin-1 beta. Agents Actions. 1988 Jun;24(1-2):130–136. doi: 10.1007/BF01968091. [DOI] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L., Steinberg T. H. Interactions of antibiotics and phagocytes. J Antimicrob Chemother. 1983 Oct;12 (Suppl 100):1–11. doi: 10.1093/jac/12.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N., Holman J. W. Entry of roxithromycin (RU 965), imipenem, cefotaxime, trimethoprim, and metronidazole into human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987 Oct;31(10):1553–1557. doi: 10.1128/aac.31.10.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Hand W. L., Francis J. B., King-Thompson N., Corwin R. W. Antibiotic uptake by alveolar macrophages. J Lab Clin Med. 1980 Mar;95(3):429–439. [PubMed] [Google Scholar]
- Koga H. High-performance liquid chromatography measurement of antimicrobial concentrations in polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1987 Dec;31(12):1904–1908. doi: 10.1128/aac.31.12.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L. Interaction of intraleukocytic bacteria and antibiotics. J Clin Invest. 1973 Jul;52(7):1673–1679. doi: 10.1172/JCI107348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. F., Martin J. R., Johnson P., Ulrich J. T., Rdzok E. J., Billing P. Erythromycin uptake and accumulation by human polymorphonuclear leukocytes and efficacy of erythromycin in killing ingested Legionella pneumophila. J Infect Dis. 1984 May;149(5):714–718. doi: 10.1093/infdis/149.5.714. [DOI] [PubMed] [Google Scholar]
- Miyachi Y., Yoshioka A., Imamura S., Niwa Y. Effect of antibiotics on the generation of reactive oxygen species. J Invest Dermatol. 1986 Apr;86(4):449–453. doi: 10.1111/1523-1747.ep12285793. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Renard C., Vanderhaeghe H. J., Claes P. J., Zenebergh A., Tulkens P. M. Influence of conversion of penicillin G into a basic derivative on its accumulation and subcellular localization in cultured macrophages. Antimicrob Agents Chemother. 1987 Mar;31(3):410–416. doi: 10.1128/aac.31.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retsema J., Girard A., Schelkly W., Manousos M., Anderson M., Bright G., Borovoy R., Brennan L., Mason R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987 Dec;31(12):1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Umar S., Dockrell H. M. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by gamma-interferon. J Immunol Methods. 1985 Sep 3;82(1):161–167. doi: 10.1016/0022-1759(85)90235-2. [DOI] [PubMed] [Google Scholar]
- Steinberg T. H., Hand W. L. Effect of phagocyte membrane stimulation on antibiotic uptake and intracellular bactericidal activity. Antimicrob Agents Chemother. 1987 Apr;31(4):660–662. doi: 10.1128/aac.31.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M., Kappus E. W., Quinn T. C. In vitro evaluation of CP-62,993, erythromycin, clindamycin, and tetracycline against Chlamydia trachomatis. Antimicrob Agents Chemother. 1987 May;31(5):811–812. doi: 10.1128/aac.31.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M., Schmitt M., Lazary S. Bovine leukocyte phagocytosis and bacteria killing monitored by intracellular acridine orange fluorescence and extracellular fluorescence quenching. Vet Immunol Immunopathol. 1987 Nov;16(3-4):185–199. doi: 10.1016/0165-2427(87)90017-1. [DOI] [PubMed] [Google Scholar]


