Abstract
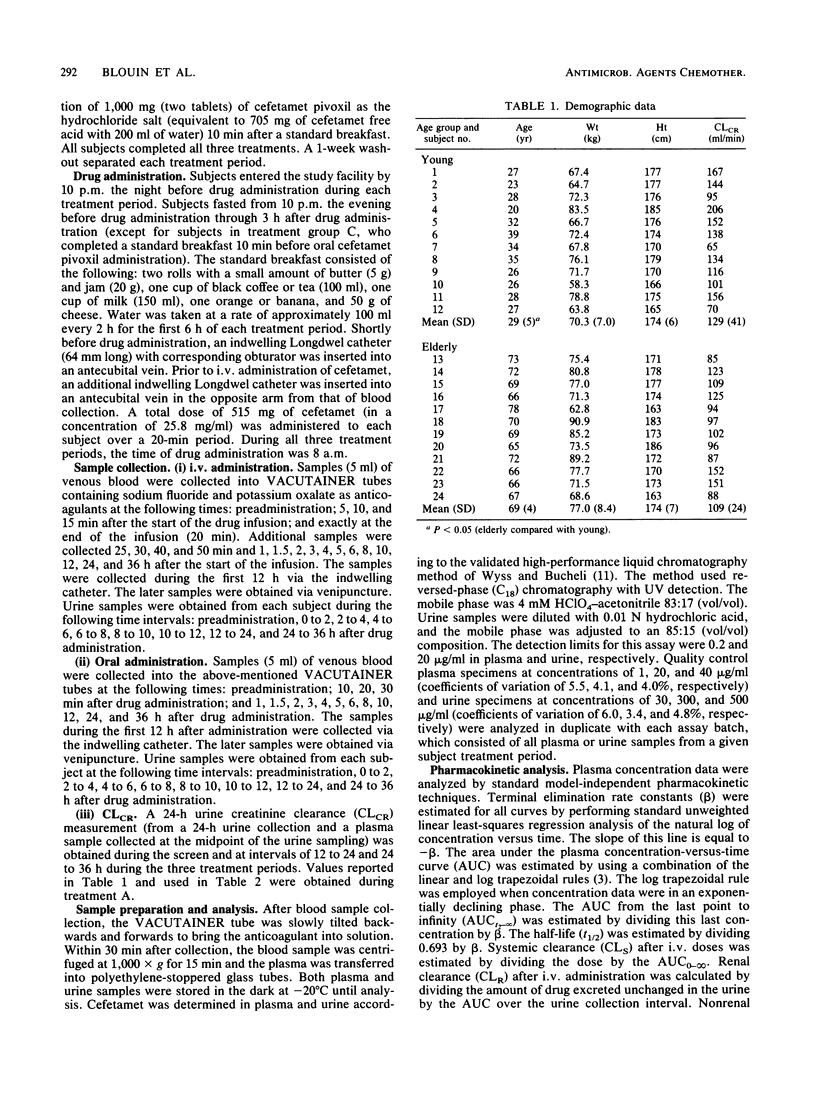
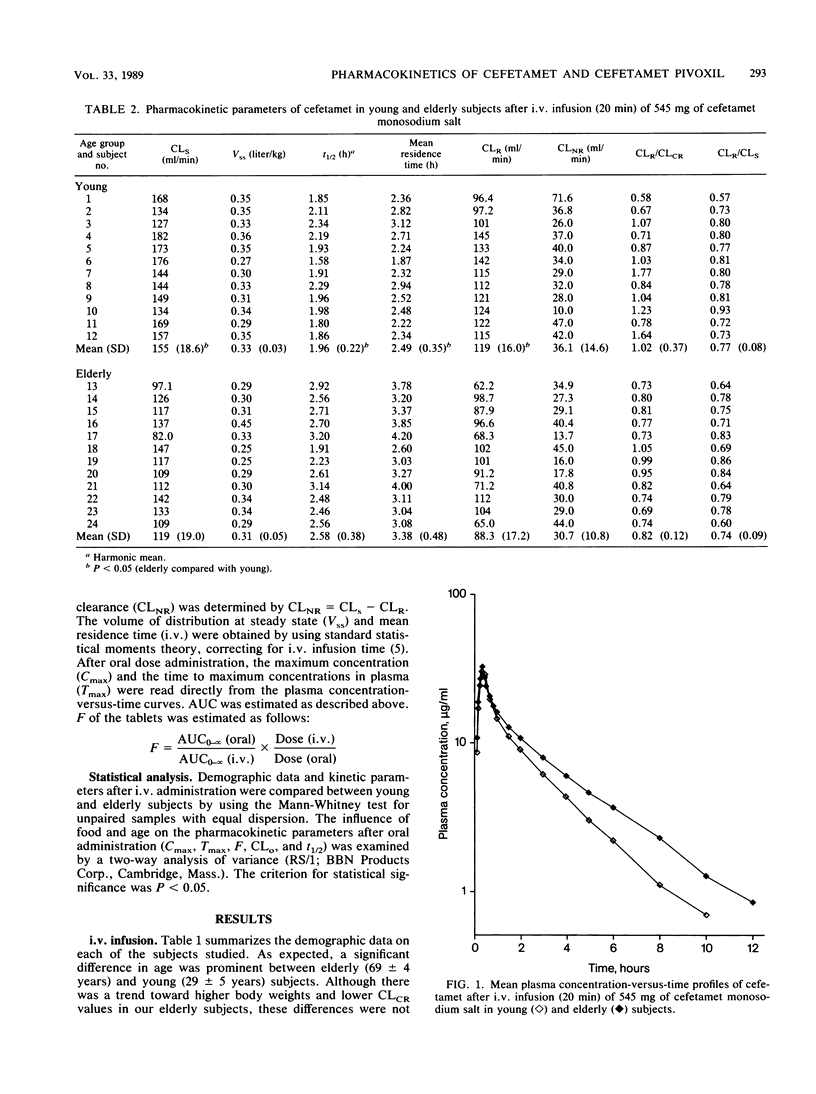
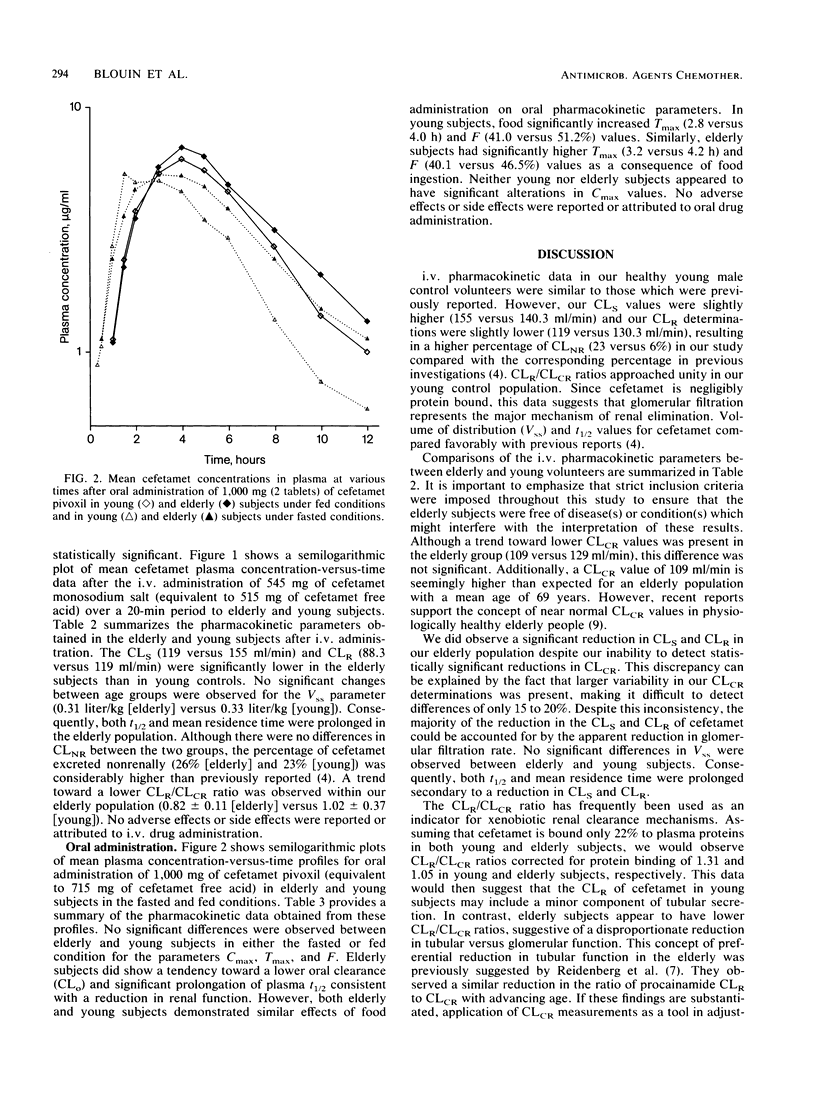
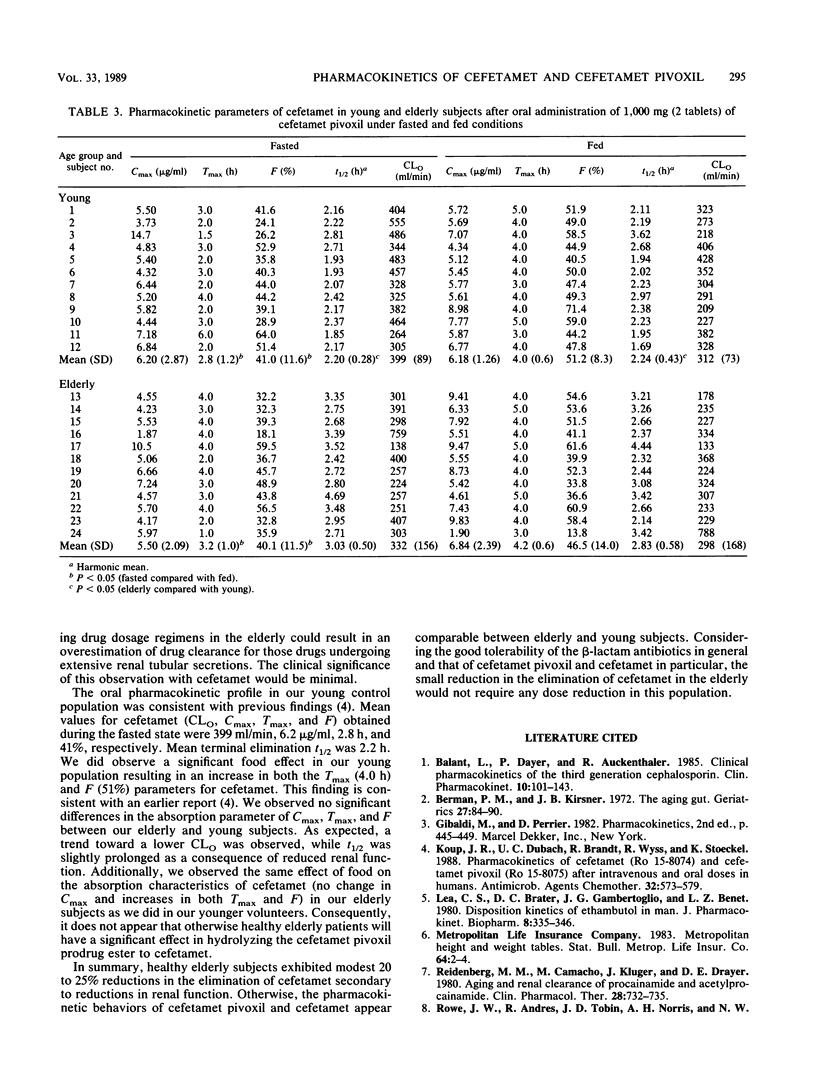
The purpose of this investigation was to evaluate the effect of advanced age on the pharmacokinetics of cefetamet and its prodrug, cefetamet pivoxil. A secondary objective of this study was to assess the effect of food on the absorption of cefetamet pivoxil in the elderly. Twenty-four healthy subjects (twelve young and twelve elderly) received (in a Latin square design) a single-dose, 515-mg infusion of cefetamet, a single 1,000-mg oral dose of cefetamet pivoxil during fasted conditions, and a single 1,000-mg oral dose of cefetamet pivoxil 10 min after a standardized low-fat breakfast. Serial blood and urine samples were collected over a 36-h period and analyzed by high-performance liquid chromatography. Intravenous and oral pharmacokinetic parameters were obtained by using model-independent techniques. The systemic clearance and renal clearance of cefetamet were significantly lower (P less than 0.05) in elderly subjects compared with in young controls after intravenous administration. No significant difference was observed in the apparent volumes of distribution at steady state between the two groups. Consequently, half-life and mean residence time were prolonged. A trend toward a lower renal clearance/creatinine clearance ratio was observed in our elderly population. Oral clearance of cefetamet was only slightly reduced in our elderly subjects, consistent with an increase in plasma half-life. Otherwise, oral pharmacokinetic parameters were comparable between elderly and young subjects. Additionally, the same effects of food were observed on the absorption characteristics of cefetamet (no change in maximum concentration of drug in plasma and an increase in both time to maximum concentration of drug in plasma and bioavailability) in our elderly subjects as in our young volunteers. Age did not appear to alter the deesterification and bioavailability of cefetamet pivoxil. We conclude that the small reduction in the elimination of cefetamet in the elderly would not require dose adjustment for this population.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balant L., Dayer P., Auckenthaler R. Clinical pharmacokinetics of the third generation cephalosporins. Clin Pharmacokinet. 1985 Mar-Apr;10(2):101–143. doi: 10.2165/00003088-198510020-00001. [DOI] [PubMed] [Google Scholar]
- Berman P. M., Kirsner J. B. The aging gut. I. Diseases of the esophagus, small intestine, and appendix. Geriatrics. 1972 Mar;27(3):84–90. [PubMed] [Google Scholar]
- Koup J. R., Dubach U. C., Brandt R., Wyss R., Stoeckel K. Pharmacokinetics of cefetamet (Ro 15-8074) and cefetamet pivoxil (Ro 15-8075) after intravenous and oral doses in humans. Antimicrob Agents Chemother. 1988 Apr;32(4):573–579. doi: 10.1128/aac.32.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S., Brater D. C., Gambertoglio J. G., Benet L. Z. Disposition kinetics of ethambutol in man. J Pharmacokinet Biopharm. 1980 Aug;8(4):335–346. doi: 10.1007/BF01059382. [DOI] [PubMed] [Google Scholar]
- Reidenberg M. M., Camacho M., Kluger J., Drayer D. E. Aging and renal clearance of procainamide and acetylprocainamide. Clin Pharmacol Ther. 1980 Dec;28(6):732–735. doi: 10.1038/clpt.1980.228. [DOI] [PubMed] [Google Scholar]
- Rowe J. W., Andres R., Tobin J. D., Norris A. H., Shock N. W. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976 Mar;31(2):155–163. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- Williams T. F. Aging or disease? Clin Pharmacol Ther. 1987 Dec;42(6):663–665. doi: 10.1038/clpt.1987.217. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Piddock L. J. In vitro activity of Ro 15-8074 and Ro 19-5247, two orally administered cephalosporin metabolites. Antimicrob Agents Chemother. 1986 Jun;29(6):1067–1072. doi: 10.1128/aac.29.6.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss R., Bucheli F. Determination of cefetamet and its orally active ester, cefetamet pivoxyl, in biological fluids by high-performance liquid chromatography. J Chromatogr. 1988 Aug 19;430(1):81–92. doi: 10.1016/s0378-4347(00)83136-9. [DOI] [PubMed] [Google Scholar]


